Development of a Eudragit-based hydrogel for the controlled release of hydrophobic and hydrophilic drugs for the treatment of wound infections
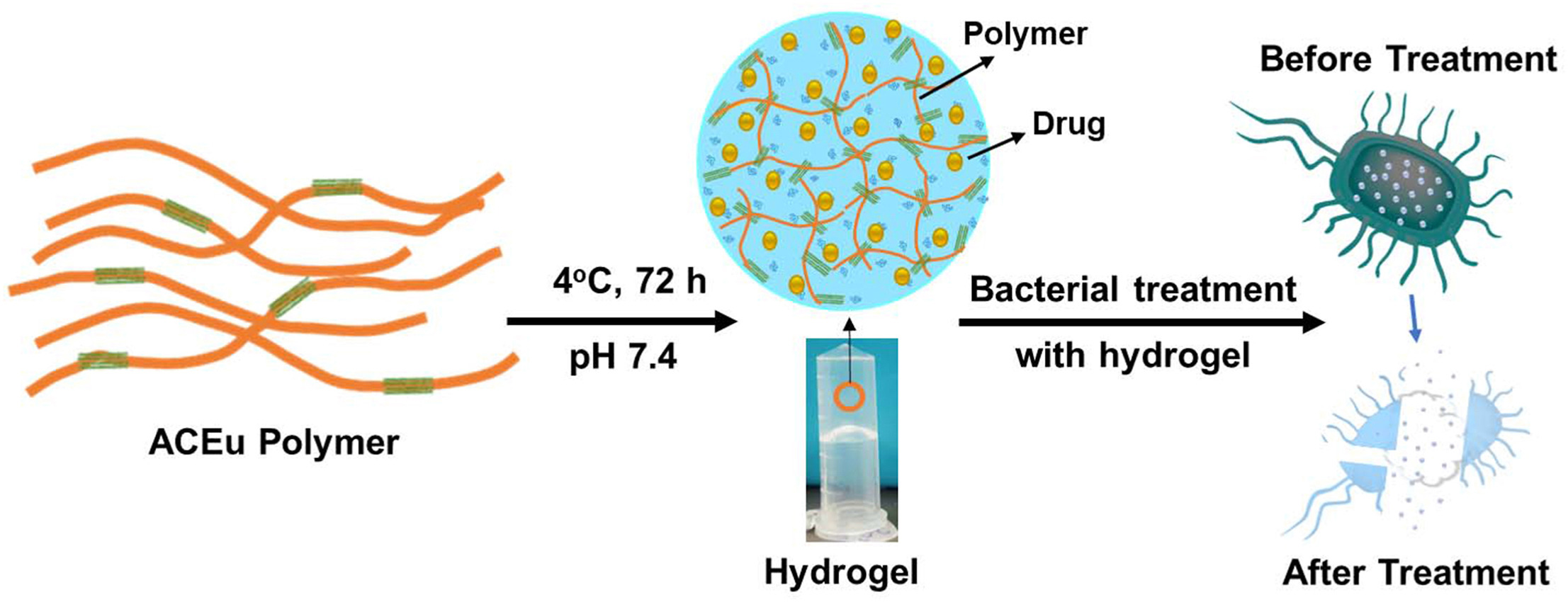
Wounds are a daily occurrence that requires wound dressings to prevent infections and promote healing. Various wound dressing types are used in clinics to prevent infections and promote healing, and dressings vary with the wound’s severity and complexity. Hydrogel-based wound dressing offers several advantages over traditional dressings, such as biocompatibility, better adhesiveness, localized therapy, and controlled drug release. Therefore, this study is focused on developing a hydrogel based on Eudragit L100, a widely used polymer in the pharmaceutical industry for its pH-sensitive properties. However, Eudragit L100 alone does not form hydrogel, thus restricting its potential for topical applications.
Therefore, adipic acid dihydrazide (ADH) was used to crosslink the Eudragit L100 polymer to synthesize the novel ADH crosslinked Eudragit L100-based polymer (ACEu polymer). The synthesized polymer was characterized using various analytical techniques to confirm crosslinking. The skin cell assay was done to verify the biocompatibility of the ACEu polymer. The ACEu polymer enabled hydrogel preparation with suitable rheological properties, controlled drug release, and efficient in vitro antibacterial activity against E. coli, P. aeruginosa, and S. aureus. Overall, the hydrogel prepared from ACEu polymer has the potential for multifunctional wound dressing that can efficiently deliver antimicrobials to treat infected wounds.
Read more here
Materials
N-hydroxysuccinimide (NHS), adipic acid dihydrazide (ADH), Dulbecco’s Modified Eagle’s Medium-high glucose (DMEM-HG), and N-(3-Dimethylaminopropyl)-N/-ethylcarbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich. Tobramycin (TBC) was purchased from Tokyo Chemical Industries Co., Ltd. (TCI). Eudragit L100 was purchased from Euro Chemo-Pharma, Malaysia. Dimethyl sulfoxide (DMSO) was purchased from MP Biomedical Inc (Singapore), and absolute ethanol (99.99%) was purchased from Fisher Scientific.
Muhammad Zahid Anwar, Himanshu Kathuria, Gigi N.C. Chiu, Development of a Eudragit-based hydrogel for the controlled release of hydrophobic and hydrophilic drugs for the treatment of wound infections, Journal of Drug Delivery Science and Technology, Volume 94, 2024, 105484, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105484.
Read also our introduction article on Topical Excipients here:


