Formulation Development of Solid Self-Nanoemulsifying Drug Delivery Systems of Quetiapine Fumarate via Hot-Melt Extrusion Technology: Optimization Using Central Composite Design
Abstract
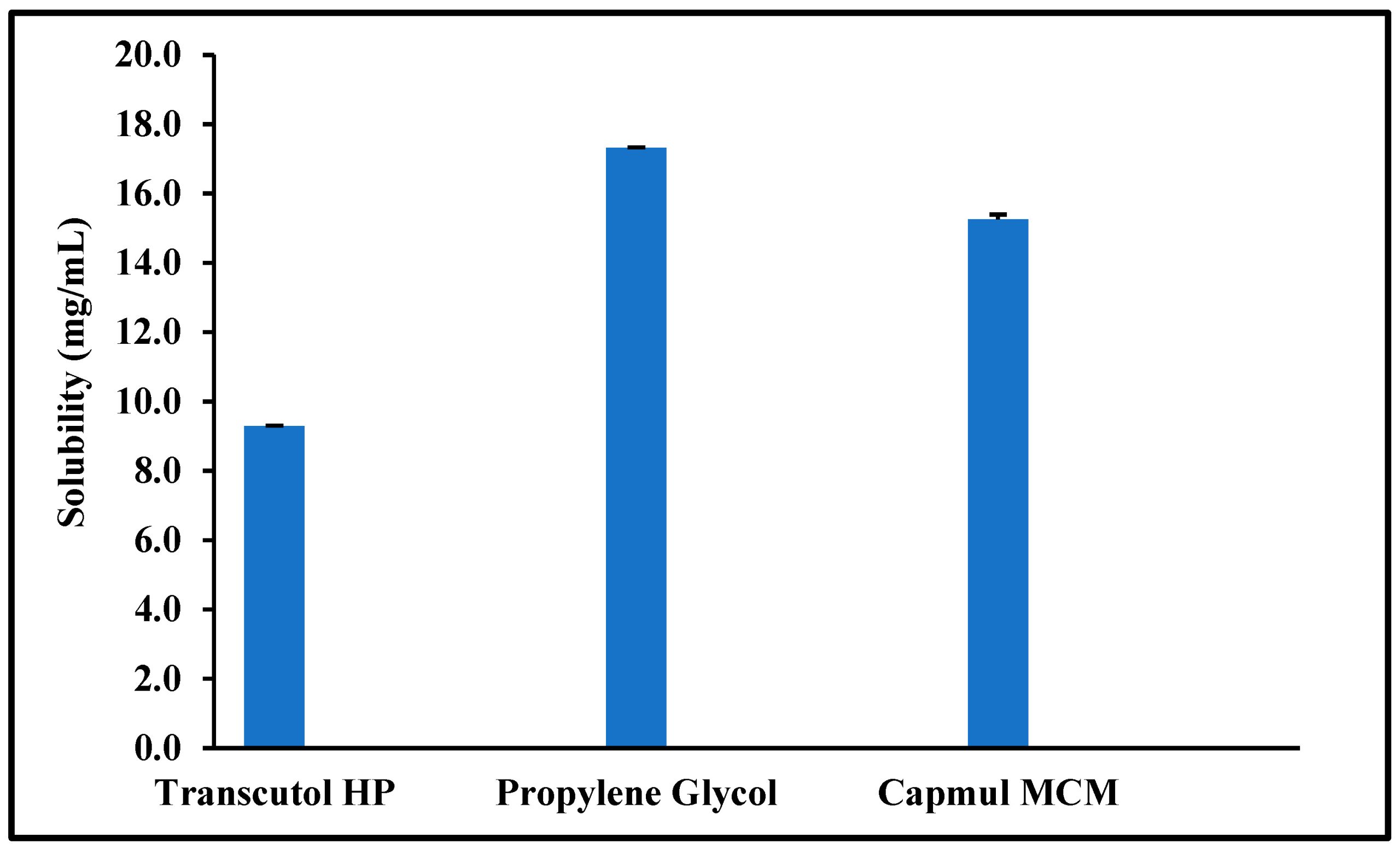
Quetiapine fumarate (QTF) was approved for the treatment of schizophrenia and acute manic episodes. QTF can also be used as an adjunctive treatment for major depressive disorders. QTF oral bioavailability is limited due to its poor aqueous solubility and pre-systemic metabolism. The objective of the current investigation was the formulation development and manufacturing of solid self-nanoemulsifying drug delivery system (S-SNEDDS) formulation through a single-step continuous hot-melt extrusion (HME) process to address these drawbacks. In this study, Capmul® MCM, Gelucire® 48/16, and propylene glycol were selected as oil, surfactant, and co-surfactant, respectively, for the preparation of S-SNEDDS. Soluplus® and Klucel™ EF (1:1) were selected as the solid carrier. Response surface methodology in the form of central composite design (CCD) was utilized in the current experimental design to develop the S-SNEDDS formulations via a continuous HME technology. The developed formulations were evaluated for self-emulsifying properties, particle size distribution, thermal behavior, crystallinity, morphology, physicochemical incompatibility, accelerated stability, and in vitro drug release studies. The globule size and emulsification time of the optimized SNEDDS formulation was 92.27 ± 3.4 nm and 3.4 ± 3.38 min. The differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD) studies revealed the amorphous nature of the drug within the formulation. There were no drug-excipient incompatibilities observed following the Fourier transform infrared (FTIR) spectroscopy. The optimized formulation showed an extended-release profile for 24 h. The optimized formulation was stable for three months (last time-point tested) at 40 °C/75% RH. Therefore, the developed S-SNEDDS formulation could be an effective oral delivery platform for QTF and could lead to better therapeutic outcomes.
1. Introduction
Oral dosage forms account for approximately 80% of the pharmaceutical market due to their ease of use, low production costs, non-sterile production, ability to be administered by the patient and the fact that high drug loading can be achieved easily [1,2]. However, poor aqueous solubility is the primary formulation development drawback of around 40% of currently available oral drugs and new chemical entities. It is estimated that about 75% of the drugs that are under development have low water solubility [3]. Classes II (low solubility and high permeability) and IV (low solubility and low permeability) in the Biopharmaceutical Classification System (BCS) are the two classes that include all poorly water-soluble drugs [4]. Many formulation strategies have emerged in recent decades to improve the oral bioavailability of poorly water-soluble drugs by increasing their apparent solubility in gastrointestinal fluids [5]. Among these methods, lipid-based formulations cover a broad spectrum of drug delivery systems that offer several benefits when administered orally, including increased drug-apparent solubility, improved permeability and decreased pre-systemic metabolism [6].
Self-nanoemulsifying drug delivery systems (SNEDDS) are a promising formulation development strategy for increasing the aqueous solubility of hydrophobic drugs. These multicomponent systems include an oil or lipid, a surfactant or a mixture of surfactants, and, optionally, a co-solvent [7,8,9]. The globule size of SNEDDS is typically within the range of 10–100 nm [10]. When SNEDDS encounter digestive fluids and gastrointestinal motility, they spontaneously convert into nanoemulsions that can solubilize the formulated drug [11]. However, most research endeavors related to SNEDDS are focused on liquid SNEDDS (L-SNEDDS). Although L-SNEDDS can be prepared very fast with rudimentary methods along with a high drug loading capacity, there are several drawbacks associated with these liquid formulations [8,12]. For example, Neoral® and Fortovase®, are soft gelatin capsules, prepared with time- and resource-intensive manufacturing techniques with a high possibility for formulation leakage outside the capsule shell. In addition, there are a few products with low market potential because of numerous obstacles such as high production costs, poor stability and mobility, the potential for drug precipitation upon dilution, the dearth of predictive in vitro methods, and the need for sophisticated manufacturing equipment [13].
Hence, novel solid dosage forms that retain the benefits of the L-SNEDDS formulation while minimizing its drawbacks are required. It is possible to convert L-SNEDDS into solid SNEDDS (S-SNEDDS) using several well-described production techniques, including adsorption to a solid carrier, wet granulation, spray drying, freeze drying, and supercritical fluid processes. The adsorption of L-SNEDDS into a solid carrier is the most widely employed formulation method for preparing S-SNEDDS at present [14]. S-SEDDS provides many benefits, including enhanced permeability, controlled drug release, and a longer GI residence time. Moreover, the production cost is minimal and the physicochemical stability is further improved [15,16]. Recently, hot-melt extrusion (HME) technology has emerged as a viable method for manufacturing S-SNEDDS. HME is the primary technology for preparing amorphous solid dispersions (ASDs) [17,18] and has also been explored in the manufacturing of pharmaceutical co-crystals [19,20], polymeric implants [21], pellets [22], and lipid nanoparticles [23]. Because the process does not require solvents and is easily scalable for continuous manufacturing, HME is an intriguing option for the advancing S-SNEDDS. In addition, HME requires less time investment than many other primitive technologies. To date, limited investigations have been reported to prepare S-SNEDDS using HME [14].
One of the recent compounds used in the treatment of schizophrenia and bipolar disorders is quetiapine, which is commercialized as a fumarate salt (QTF). The atypical antipsychotic QTF is a dibenzo thiazepine derivative that was approved by the Food and Drug Administration (FDA) in 1997. QTF is classified as a class II drug under the BCS. QTF’s poor solubility and significant hepatic first-pass metabolism contributed to its low oral bioavailability (9%) [24,25].The current research focuses on continuously manufacturing the S-SNEDDS of QTF using HME technology for the first time. The developed systems could improve drug solubility, promote lymphatic transport to reduce first-pass metabolism, and extend QTF release following oral administration. The effect of the formulation variables on the performance of developed S-SNEDDS was investigated using a central composite design (CCD). The developed systems were optimized based on globule size and emulsification time. The optimized formulation was evaluated for in vitro release, drug-excipient incompatibility, thermal behavior, and stability studies.
2. Materials and Methods
2.1. Materials
QTF was purchased from SPECTRUM® CHEMICAL NFG CORP (New Brunswick, NJ, USA). The lipid excipients Gelucire 48/16, and Gelucire 44/14 were kindly gifted by Gattefossé (Paramus, NJ, USA). Hydroxypropyl cellulose (Klucel™ EF; HPC-EF) was gifted by Ashland Global Chemicals Company (Burlington, NJ, USA). Polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer (Soluplus®) was received as a generous gift from BASF Chemical Co. (Ludwigshafen, Germany). Capmul MCM (Medium Chain mono and di-glycerides) was gifted by ABITEC Corporation (Columbus, OH, USA). Propylene glycol and other excipients used for screening studies were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents utilized in the current investigation were of analytical grade and were purchased from Fischer Scientific (St. Louis, MO, USA). The size 00 hard gelatin capsules were procured from Total Pharmacy Supply Inc. (Arlington, TX, USA).
Download the full study as PDF here: Formulation Development of Solid Self-Nanoemulsifying Drug Delivery Systems of Quetiapine Fumarate via Hot-Melt Extrusion Technology: Optimization Using Central Composite Design
or read it here
Uttreja, P.; Youssef, A.A.A.; Karnik, I.; Sanil, K.; Narala, N.; Wang, H.; Elkanayati, R.M.; Vemula, S.K.; Repka, M.A. Formulation Development of Solid Self-Nanoemulsifying Drug Delivery Systems of Quetiapine Fumarate via Hot-Melt Extrusion Technology: Optimization Using Central Composite Design. Pharmaceutics 2024, 16, 324. https://doi.org/10.3390/pharmaceutics16030324

