The Impact of Formulation and Freeze Drying on the Properties and Performance of Freeze-Dried Limosilactobacillus reuteri R2LC
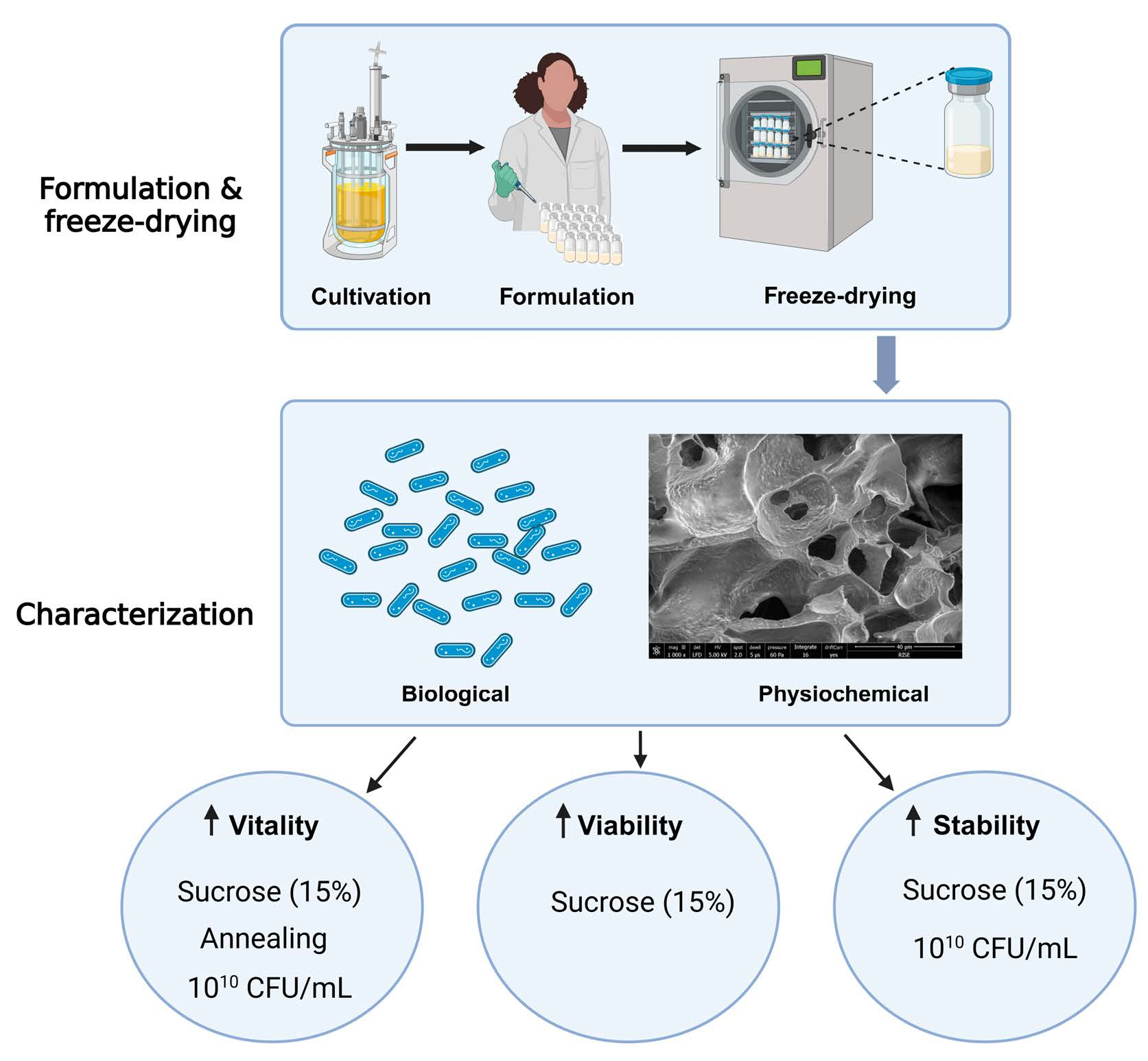
Freeze drying is a commonly used method for preserving probiotic bacteria and live biotherapeutic products. Before drying, the bacterial cells are formulated with a lyoprotectant, and the design of these two process steps are crucial to achieve a high-quality product. There are several factors that may affect the biological and physicochemical properties of the freeze-dried cells and we have used a Design of Experiment approach to investigate the effects of formulation and freeze-drying parameters on properties and performance of Limosilactobacillus reuteri R2LC. The biological characteristics of the dried bacteria were evaluated by measuring cell survival, metabolic activity and stability, and physicochemical characteristics were studied using visual inspection, differential scanning calorimetry (DSC), scanning electron microscopy (SEM), and analysis of residual moisture content and bacterial aggregation.
A comparison between the lyoprotectants trehalose and sucrose showed that the latter gave better freeze-drying survival, metabolic activity, and storage stability. We also want to highlight that there was a correlation between bacterial concentration, metabolic activity, and aggregation of bacteria, where a higher concentration (1010 CFU/mL) resulted in both higher metabolic activity and aggregation. Several other process and formulation factors affected both the biological and physicochemical properties of freeze-dried L. reuteri R2LC and it could be concluded that care must be taken to develop a production method that generates a product with high and consistent quality. These results may, or may not, be strain specific.
1. Introduction
One way to promote the efficiency of freeze drying is to include an annealing step in the process, which involves keeping the product at a temperature above the glass transition temperature for about 15 min, resulting in increased ice crystal growth and ultimately lower water content in the dried product [21]. Previously, it has been shown that annealing with trehalose phosphate as the lyoprotectant is effective in achieving an efficient drying and increased stability of a strain of Lactobacillus acidophilus [22]. As a general goal, low water activity (<0.2) and residual water content (<5%) are required to obtain robust and stable freeze-dried probiotics [23].
The aim of this study was to investigate the influence of formulation and annealing on biological properties and physicochemical characteristics of freeze-dried Limosilactobacillus reuteri R2LC. This is a well-studied strain that has been shown to ameliorate acetic acid or DSS-induced colitis in rats [24] and mice [25]. In a recent paper, R2LC has been shown to mediate its anti-inflammatory effect and modulate the intestinal microbiota by transmitting probiotic signals to immune cells present in Peyer’s patches [26]. The strain also has antimicrobial activity mediated by a polyketide [27]. We used a Design of Experiment (DOE) approach to evaluate the effects of different lyoprotectants (sucrose and trehalose) with concentrations between 10 and 20%, different bacterial concentrations (109 and 1010 CFU/mL), and annealing. The biological properties analysed included viability, metabolic activity, and stability, and the physicochemical characteristics analysed included water content, matrix structure, cake appearance, glass transition temperature, and aggregation of the bacteria.
2. Materials and Methods
2.1. Experimental Design
To investigate the impact of formulation and freeze drying on the biological and physicochemical properties of R2LC, a Design of Experiments (DoE) approach was taken. In this study, four different factors were investigated: type of lyoprotectant (sucrose and trehalose), lyoprotectant concentration (10, 15, and 20%), bacterial concentration (109 and 1010 CFU/mL), and the freeze-drying process (with and without annealing). MODDE version 13 (Sartorius Data Analytics, Umeå, Sweden) was used to generate a full factorial screening study with 24 independent runs.
Download the full article as PDF here: The Impact of Formulation and Freeze Drying on the Properties and Performance of Freeze-Dried Limosilactobacillus reuteri R2LC
or read it here
Tyagi, N.; Gidlöf, Z.; Osanlóo, D.T.; Collier, E.S.; Kadekar, S.; Ringstad, L.; Fureby, A.M.; Roos, S. The Impact of Formulation and Freeze Drying on the Properties and Performance of Freeze-Dried Limosilactobacillus reuteri R2LC. Appl. Microbiol. 2023, 3, 1370-1387. https://doi.org/10.3390/applmicrobiol3040092

