A toolbox for mimicking gastrointestinal conditions in children: Design and evaluation of biorelevant dissolution media for mimicking paediatric gastric- and small intestinal conditions
Abstract
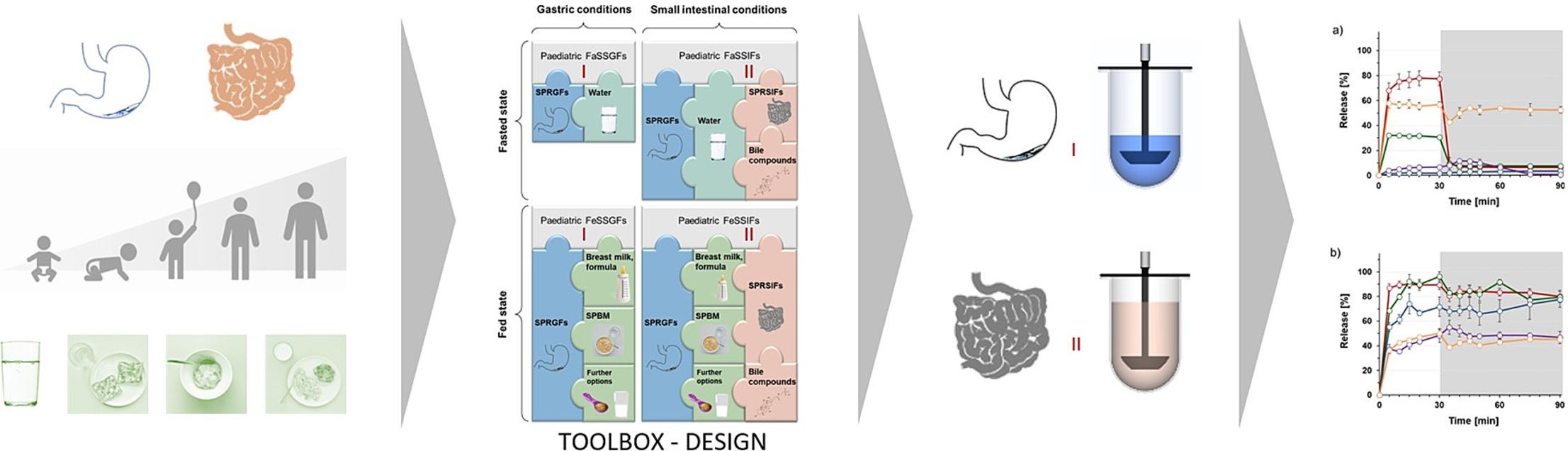
The goal of the present work was to develop an in vitro toolbox to evaluate the oral administration of dosage forms to children of different age groups and under different administration conditions (fasted/fed). Based on current data on the gastrointestinal physiology of children, a set of new biorelevant media was designed to mimic the composition and physicochemical properties of resting gastric and resting small intestinal fluid in children of different age groups.
In addition, guidelines were developed on how to generate fasted and fed state gastric and small intestinal fluids by combining these media with age-specific drinking volumes or portions of already established simulated paediatric breakfast meals, respectively. These fluids can simulate the conditions in the paediatric stomach and small intestine after administration of a dosage form in the fasting state or after a breakfast. The in vitro toolbox was evaluated using the example of pre-school children with a total of five paediatric medicines. Results from the corresponding set of in vitro studies highlight the importance of addressing patient-specific characteristics rather than downscaling existing adult in vitro models.
Introduction
Clinical trials are an important element in the development process of safe and effective drug products. They are usually conducted in healthy adults or in selected adult patients suffering from the respective disease and therefore provide information on the in vivo performance of the dosage form in question in average adult patients [1]. However, when it comes to developing drug products for children, the situation is much more difficult. To ensure safe drug therapy in children, it is necessary either to ensure that results from clinical trials in adults can be translated to children or to conduct clinical trials in children. As a consequence of ethical concerns, formulation exploration studies are rarely conducted in paediatric populations. To facilitate the development of safe and effective medicines for children, various extrapolation approaches are being pursued to avoid unnecessary studies in children [2], [3]. However, to which extent results from studies conducted in adults can be extrapolated to children is a topic for debate. On one hand, extrapolation of efficacy can promote paediatric drug development if certain criteria such as similar disease progression and a similar exposure–response relationship are met [3], [4]. On the other hand, children are a highly vulnerable patient group and various factors may limit or preclude extrapolation, such as gaps in knowledge on the pathophysiology of the disease, and differences in clinical outcomes between study- and target population [2]. In addition, it is likely that the heterogeneity of children in terms of maturation and growth may lead to differences in pharmacokinetics, and therefore the exposure in children of different ages cannot simply be extrapolated from adult data but should be determined by clinical studies in the target population [2], [4], [5]. Thus, with the aim of protecting children from unnecessary in vivo studies, there is a great deal of interest in the development and application of alternative tools, such as in vitro and in silico models. Both, in vitro dissolution tests and physiology-based pharmacokinetic (PBPK) models play an important role in estimating the in vivo performance of dosage forms and are increasingly used for predicting pharmacokinetic (PK) profiles or behaviour of oral dosage forms in adults. It would be desirable to be able to use such models in paediatric drug development as well, to reduce the number of clinical trials in children and the risks associated with such trials.
Biorelevant in vitro test methods have emerged as valuable tools in the field of adult drug delivery when aiming to predict the in vivo performance of orally administered dosage forms in the gastrointestinal (GI) tract. To obtain the best possible in vitro-in vivo correlation (IVIVC), biorelevant in vitro test methods must simulate as closely as possible the luminal conditions that influence the timing, site, rate, and extent of drug release. Consequently, when aiming to predict in vivo performance based on data from biorelevant in vitro dissolution/drug release experiments, various aspects such as volumes and composition of the luminal contents as well as GI motility and passage times in the different GI segments, must be considered in the test design. To which extent these parameters must be addressed, and which GI segments will be relevant for the in vitro test design is depending on the drug and the formulation concept of the dosage forms to be tested [6]. As already stated, several in vitro release methods focusing on different parameters have already been developed with the intention of providing a good estimate of the in vivo performance of orally administered dosage forms in the GI tract of adults [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Whether a downscaled version of these models could provide a good predictive power for release behaviour in children is questionable. Children are a very heterogeneous population with a whole range of anatomical and physiological differences both within the population and compared to adults. Especially in very young children, not only the physiology of the GI tract but also the diet differs significantly from that of adults, which may have a considerable impact on the in vivo performance of orally administered drugs. Consequently, there is an urgent need for the development of biorelevant paediatric in vitro dissolution/drug release tests, which will hereinafter be collectively referred to as in vitro dissolution tests. The in vitro release profiles obtained by such in vitro test methods would allow estimation of in vivo drug release, and the data obtained could be integrated into PBPK models for better prediction of oral drug absorption. So far, a few in vitro studies aiming to address differences in GI physiology and administration conditions in children have been published [22], [23], [24], [25], [26], [27]. To date, only one study has addressed the development of dissolution media that simulate the composition of luminal contents in children [24]. While the composition of biorelevant media for simulating conditions in adults has been optimized in the past [28], the development of media for simulating GI conditions in children is still in its infancy.
In 2016, Maharaj et al. proposed dissolution media for addressing the GI environment of neonates and infants in the fasted state and after milk or soybean formula feeding [24]. While the media proposed in this study focused on mimicking intraluminal conditions in very young infants, there are a number of other age groups and administration conditions for which appropriate test models need to be developed. Since 2016, new results from in vivo studies investigating the properties of GI fluids in children have also been published [29], [30], [31]. It is evident that biorelevant dissolution media need to be developed based on the latest and most reliable information on paediatric GI parameters and tailored to all individual age groups of children. In this context, both the properties of resting fluids in the fasting GI tract of children and the composition and properties of the intraluminal contents after consumption of water or typical meals should be considered. However, this objective raises the question of what typical meals are consumed by children and whether the same meal can be considered relevant to the “best” or “worst” administration conditions in all paediatric age groups. Especially in older children, the diet is much more variable than in neonates and infants, but still cannot be compared to that of adults [32]. When mimicking fed-state gastric conditions in adults, milk and Ensure® Plus are often used for mimicking the composition and physicochemical properties of a light meal or a standardized high-fat meal such as the FDA standard breakfast, respectively [12], [28]. However, whether the use of milk and Ensure® Plus is also suitable to simulate postprandial conditions in the GI tract of children is questionable [32]. Even if smaller portions were to be used, younger children are unlikely to eat a breakfast with the composition of an FDA standard breakfast.
The aim of the present work was to complement the set of available paediatric dissolution media. As a first step, Simulated Paediatric Resting Gastric Fluids (SPRGFs) and Simulated Paediatric Resting Small Intestinal-Fluids (SPRSIFs) should be developed to mimic fasting GI conditions (i.e., gastric and small intestinal fluid compositions and volumes after an overnight fast and before administration of any fluid or food) in children of different age groups. These media should account for the heterogeneity of this population and provide the basis for an in vitro toolbox to simulate intraluminal GI conditions in children of different age groups and can then be specifically combined with age-appropriate drinking volumes of water and age-specific meals to mimic conditions of administration of a dosage form in both the fasting state and after a meal. Once completed, the application of this toolbox in in vitro formulation assessment should allow good prediction of in vivo release performance for each age group and administration condition. Recently, various Simulated Paediatric Breakfast Media (SPBM) have been developed. These SPBM can be used in an in vitro dissolution setup to mimic the composition and physicochemical properties of the first meal of the day, before or after which the dosage form is likely to be administered [32]. Provided that SPRGFs and SPRSIFs could be established to simulate the fluid volumes and compositions in the “empty” stomach and small intestine of children of different age groups, they can be combined with age-appropriate portions of these SPBMs to simulate gastric and small intestinal contents in these children after administration of oral dosage forms with an age-appropriate breakfast [32]. With the aim of demonstrating the importance of developing child-specific in vitro dissolution assays, age-appropriate administration conditions should also be considered for the in vitro experiments. Pre-school children (psc) were used as the example age group. For comparison, typical administration conditions, especially postprandial administration conditions, as used in clinical trials with adults should be considered to support the hypothesis that downscaling from typical administration conditions in adults is likely not suitable to draw conclusions on typical administration conditions in children of different age groups.
Read more here
Lisa Freerks, Tina Arien, Claire Mackie, Sabine Inghelbrecht, Sandra Klein, A toolbox for mimicking gastrointestinal conditions in children: Design and evaluation of biorelevant dissolution media for mimicking paediatric gastric- and small intestinal conditions, European Journal of Pharmaceutics and Biopharmaceutics, Volume 193, 2023, Pages 144-157, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.10.011.
Read also other articles to paediatric topics:
- Small patients, big challenges: navigating pediatric drug manipulations to prevent medication errors – a comprehensive review
- Extemporaneous preparation of paediatric oral formulations with sildenafil citrate
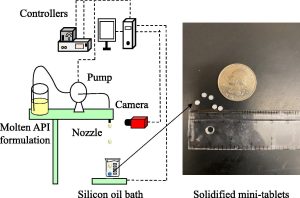
- Manufacturing pharmaceutical mini-tablets for pediatric patients using drop-on-demand printing