Lasmiditan Nanoemulsion Based In Situ Gel Intranasal Dosage Form: Formulation, Characterization And In Vivo Study
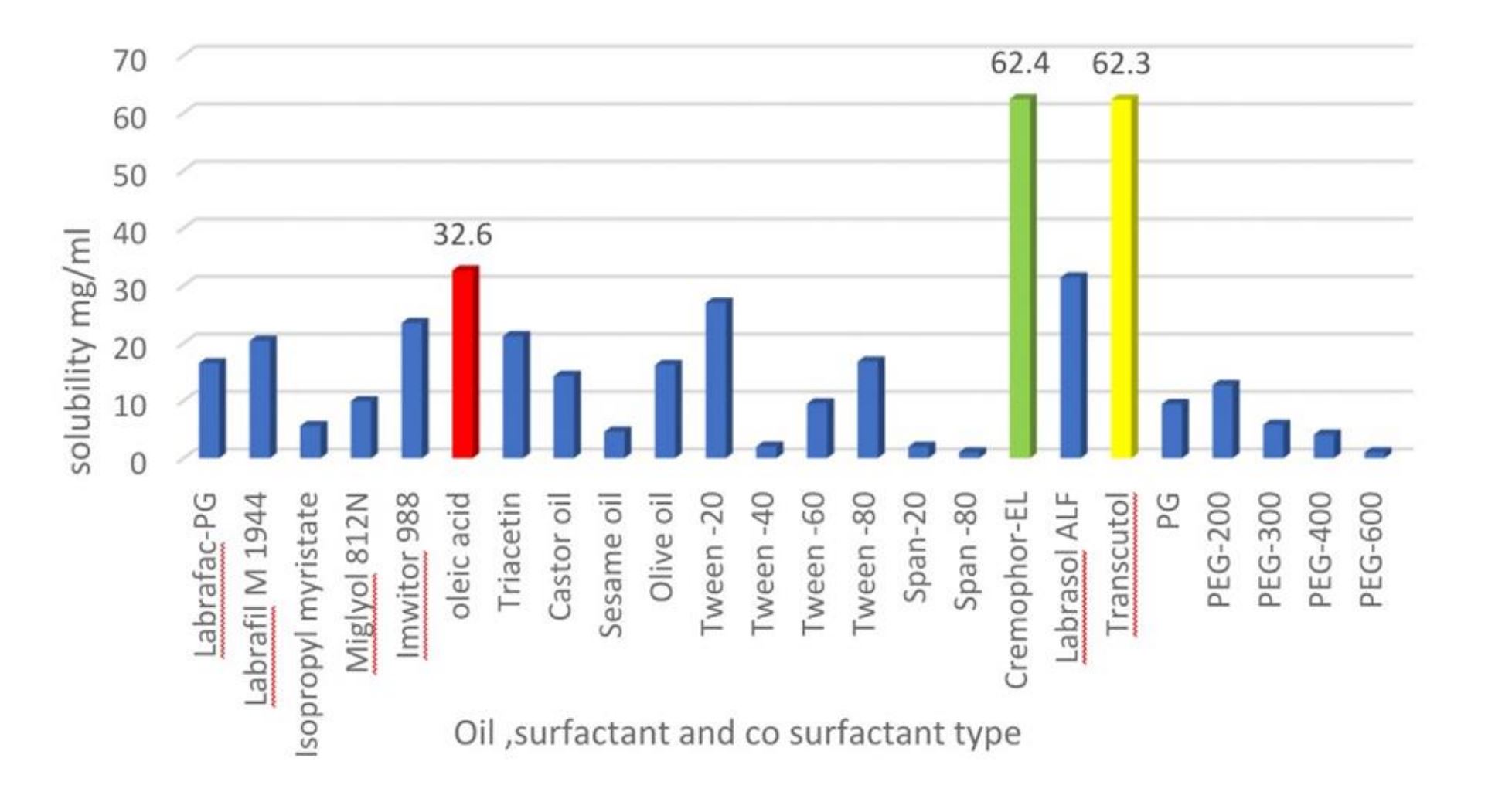
This study aimed to formulate lasmiditan (LAS) as a nanoemulsion in situ gel (NEIG) by utilising nanotechnology in order to escape the problems associated with the poor oral bioavailability of the drug. A study regarding the LAS solubility in different oils, surfactants and co-surfactants, was determined. Different nanoemulsion (NE) formulations were prepared depending on the pseudo-ternary phase diagrams, which were first constructed. Visual characterization, thermodynamic stability study and droplet size were determined for the prepared NEs. Muco-ciliary clearance can be overcome with the in situ gelling agent carbopol 934, a pH-sensitive polymer that was selected to increase the residence time on the nasal mucosa. The gel strength, pH, gelation time and viscosity were predicted for the prepared NEIG. In vitro release and ex vivo nasal permeation were measured for both NE and NEIG formulations.
Formula coded-15 (F15) with a droplet size of 58.4 nm provided a maximum in vitro as well as ex vivo permeation to be considered as a selected one, to which 0.5% carbopol 934 was added for the preparation of NEIG2 that exerts comparable release and permeation values as F15 with more residence time in order to overcome the normal nasal physiological clearance. Finally, LAS as NEIG2 was subjected to an in vivo study as a promising intranasal novel formula that exerts rapid onset of action (Tmax 0.75 hours) accompanied by higher bioavailability (2.5 folds) and tissue permeation (4 folds) relative to the oral dosage form (8% aqueous LAS suspension) was considered.
Download the full article as PDF here Lasmiditan Nanoemulsion Based In Situ Gel Intranasal Dosage Form
Materials
Materials Lasmiditan was purchased from Yongchi Chemical Technology Co., LTD., China. Labrafil M 1944, Labrafac PG, Labrasol ALF, isopropyl myristate, Transcutol and Cremophor® EL were purchased from Gattefosse (France). Imwitor® 988 and Miglyol 812 N were purchased from IOI Oleochemical GmbH, Germany. Oleic acid (OA), triacetin, propylene glycol (PG) and carbopol 934 were purchased from Central Drug House (CDH®). Castor, olive and sesame oils were purchased from Now Food, USA. Potassium dihydrogen phosphate and disodium hydrogen phosphate, Tween 20, 40, 60, 80, Span 20, 80, Polyethylene glycol (PEG) 200, 300, 400, 600 were all purchased from Hi Media Laboratories Pvt. Ltd., India. Methanol was purchased from Loba Chemie Pvt. Ltd., India. A Millipore filter syringe was purchased from Chm Lab, Spain. Dialysis Bag MD34-5M, Wide flat: 34 MM, Mw: 8000 – 14000 D was purchased from MYM Biomedical Technology Company Limited, USA. Deionized water (DD), the rest of the chemicals and reagents were of analytical grade.
Saba H. Jaber, Nawal A. Rajab, Lasmiditan Nanoemulsion Based In Situ Gel Intranasal Dosage Form: Formulation, Characterization And In Vivo Study, FARMACIA, 2023, Vol. 71, 6, Manuscript received: August 2023, Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq

