Effect of tableting temperature on tablet properties and dissolution behavior of heat sensitive formulations
Abstract
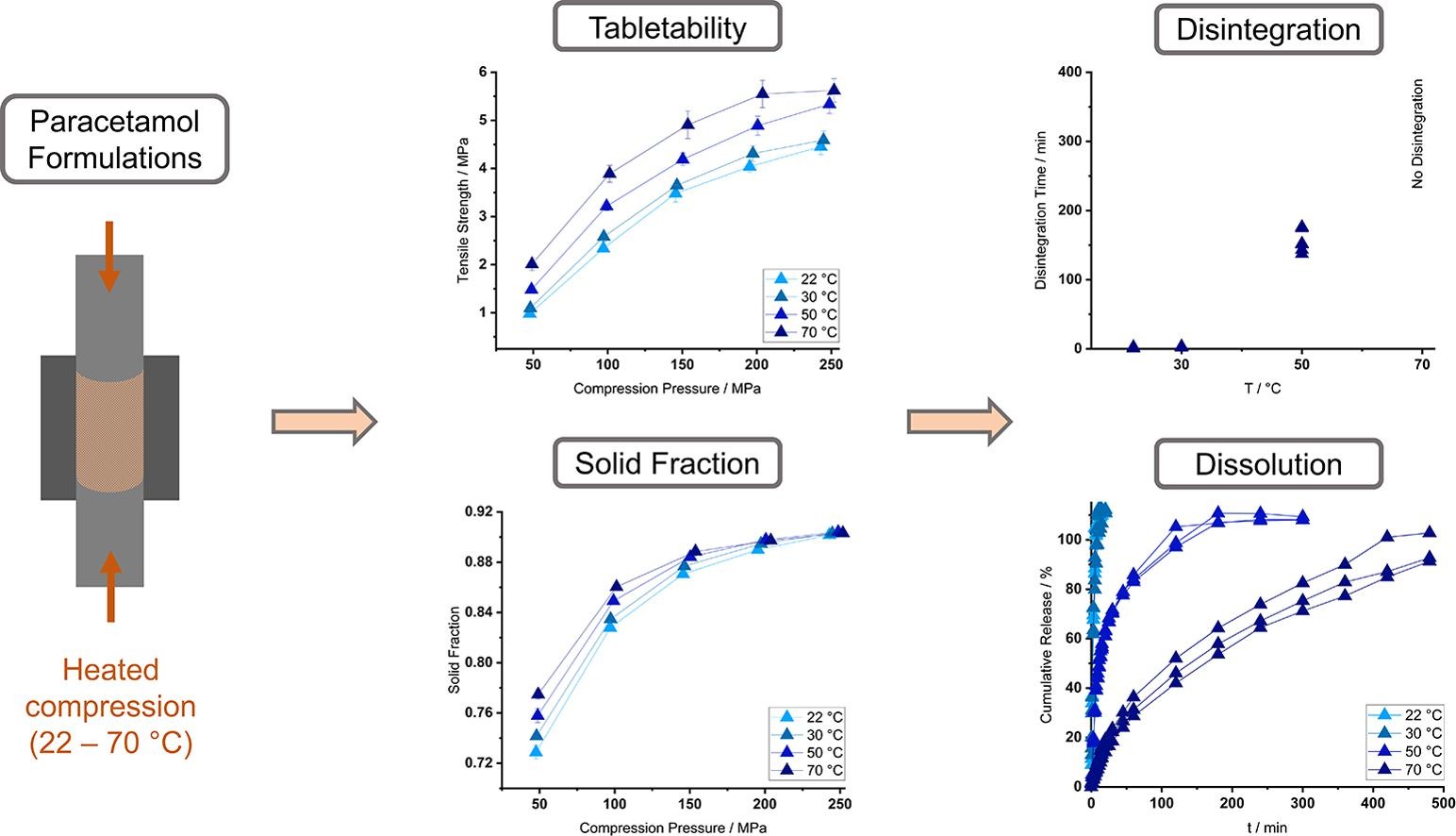
The tableting process involves the conversion of mechanical to thermal energy. This study evaluated the influence of temperature on the tableting behavior of formulations with different compositions. The tableting machine was equipped with a thermally controlled die to mimic the heat evolution from tableting on an industrial scale. Six formulations containing binders with a comparably low glass transition temperature were examined. Besides the polymer type and concentration, the filler was varied. Paracetamol was chosen as the model active pharmaceutical ingredient. The investigation included alterations in tabletability, disintegration and dissolution. Elevated temperatures led to an enhanced tabletability. The polymer type and concentration were decisive for the extent of alterations.
The variation of the filler composition played a minor role due to the high melting points of its components. The results were confirmed in disintegration and dissolution studies. A high binding capacity and a low glass transition temperature resulted in a stronger delay of disintegration. The dissolution was sustained. Increased concentrations of the binding polymer enhanced the effect. If the tableting behavior of a formulation is changed by elevated temperatures during formulation development and production, a change of the binder type or concentration should be considered to ensure a reproducible tablet quality.
Introduction
The compression of powders into tablets involves the generation of heat. Besides the elastic and plastic deformation of materials as well as fragmentation, friction occurs (Hanus and King, 1968, Zavaliangos et al., 2008). It arises from the interaction between powder particles, particles and the die-wall and between the machine components. If the physicochemical properties of an active pharmaceutical ingredient (API), excipient or a complete formulation are thereby altered, the process parameters and tablet characteristics may vary. This might lead to challenges during formulation development and on a full-production scale, if reproducible results can no longer be ensured. The subsequent batch release might be compromised. The heat generation from tableting has been subject to numerous studies (Bechard and Down, 1992, Ketolainen et al., 1993, Wurster et al., 1995, Picker-Freyer and Schmidt, 2004, Zavaliangos et al., 2008). A detailed overview has recently been published (Grumann, Klinken et al., 2022).
During manufacturing, the release of a produced batch includes the assessment of critical tablet quality attributes. The mechanical strength of tablets is reflected in the tabletability analysis, where their tensile strength is related to the compression pressure. The tensile strength is also influenced by the solid fraction, which is the counterpart to a compact’s porosity. The solid fraction can significantly influence a tablet’s disintegration and dissolution behavior. A fast disintegration triggers the rapid drug release from a dosage form (Zhao and Augsburger, 2005, Markl and Zeitler, 2017, Nickerson et al., 2018). For the disintegration process, the solvent needs to be able to penetrate into the tablet. Depending on the used solvent and excipients, the tablet components are hydrated to the point of swelling or the dissolution of polymers (Wan et al., 1991, Reynolds et al., 1998, Maggi et al., 2002). The underlying formulation might therefore determine to which kinetic an API is released besides its own dissolution rate (Ei-Arini and Leuenberger, 1995, Costa and Sousa Lobo, 2001). As dissolution is a prerequisite for the bioavailability of a drug, its description and prediction by mathematical models is desirable (Costa and Sousa Lobo, 2001, Siepmann and Peppas, 2001, Siepmann and Siepmann, 2008).
A formulation’s response to heat is strongly dependent on its components and their respective properties. Amorphous or partially crystalline polymers are of particular relevance and can be used as binding agents. Their glass transition temperature (Tg) acts as a critical parameter. When the Tg of a polymer is approached, the additional thermal energy allows for enhanced chain movement, which reduces its rigidity (Cowie and Arrighi, 2007). The increased molecular mobility facilitates the deformation of a material during compaction. If the Tg is sufficiently low, its reversible transgression during tableting can alter tablet characteristics. The enhanced deformability triggers a decrease in porosity, which results in a higher mechanical strength of a compact (Pilpel, Britten et al., 1991). The correlation between the Tg of a material and varying tablet characteristics upon temperature rise has been subject to several investigations (Van der Voort Maarschalk et al., 1997, Picker, 2003, Partheniadis et al., 2020, Grumann et al., 2022).
Besides the types of components used, tablet characteristics are also affected by their respective concentration. If the concentration of one substance is changed, the behavior of the whole system can be altered. This phenomenon is reflected in the percolation theory. It describes the cluster formation of randomly occupied sites within a lattice (Leuenberger et al., 1987, Holman and Leuenberger, 1988, Stauffer and Aharony, 2018). Clusters can be defined as established bonds between particles of nearest neighbor sites (Holman and Leuenberger, 1988, Stauffer and Aharony, 2018). A percolating cluster and therefore a coherent lattice can be expected above a critical concentration, which is the particles’ percolation threshold (Stauffer and Aharony, 2018). It is the prerequisite for compact integrity (Holman and Leuenberger, 1988). Above the percolation threshold, the concentration of one or more substances can command the behavior of the system by creating a continuous network (Leuenberger, Rohera et al., 1987). For instance, it has been shown for binary mixtures composed of an active substance and a disintegrant, that some excipients might coat the active substance coherently if their critical concentration is exceeded. The formed excipient clusters are able to decelerate drug release (Leuenberger, Rohera et al., 1987). The behavior is dependent on the components of a formulation.
Although the effect of temperature during tableting has been investigated in several works, studies on the behavior of complete formulations are scarce (Britten and Pilpel, 1978, Pilpel et al., 1991). Previous research mostly put a focus on pure materials (Picker, 2003, Picker-Freyer and Schmidt, 2004, Cespi et al., 2013) or binary mixtures (Casettari et al., 2016, Grumann et al., 2022). Tablet properties were often only considered in isolation, whereby the Tg of the investigated materials was not particularly low. Casettari, Bonacucina et al., (2016) investigated, among other things, the tabletability and dissolution behavior of binary mixtures containing acetyl salicylic acid and polyethylene oxide (PEO) upon temperature rise. While the tabletability was clearly enhanced for higher tableting temperatures, there was no effect of temperature visible during the dissolution studies. However, the lowest melting point of PEO (64.4 °C) was above the maximum tableting temperature of 50 °C. In a study by Van der Voort Maarschalk, Zuurman et al., (1997) polymers with different Tgs were synthesized and subsequently compressed. While this offered valuable insights into the alteration of yield pressure, elastic modulus and the resulting tablet porosity, the investigations are limited by the absence of an API or other typical formulation components.
The aim of this work was to determine the tableting behavior of formulations containing heat sensitive polymers upon temperature rise. Hence, the Tg of the investigated polymers used as binders was within the applied temperature range. The dependency of tablet quality attributes on the polymer type and concentration was evaluated.
Read more here
Materials
Paracetamol (PCM/Paracetamol, Atabay, Turkey) was selected as a model active pharmaceutical ingredient (API) for all formulations Fx (Table 1). For the formulations F1-F4, the binder content of 10 % was kept constant. For F1 and F2, a copolymer of N,N-dimethylaminoethyl methacrylate, methyl methacrylate and butyl methacrylate was used as a binding agent (E/Eudragit® E PO, Evonik Industries, Germany).
Hanna Dorothea Grumann, Peter Kleinebudde, Effect of tableting temperature on tablet properties and dissolution behavior of heat sensitive formulations, International Journal of Pharmaceutics, Volume 648, 2023, 123603, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123603.
Read also more on Disintegrants – Pharmaceutical Excipients here:


