HiSORAD™ – Solving content uniformity issues in ODT prepared by direct compression
Introduction
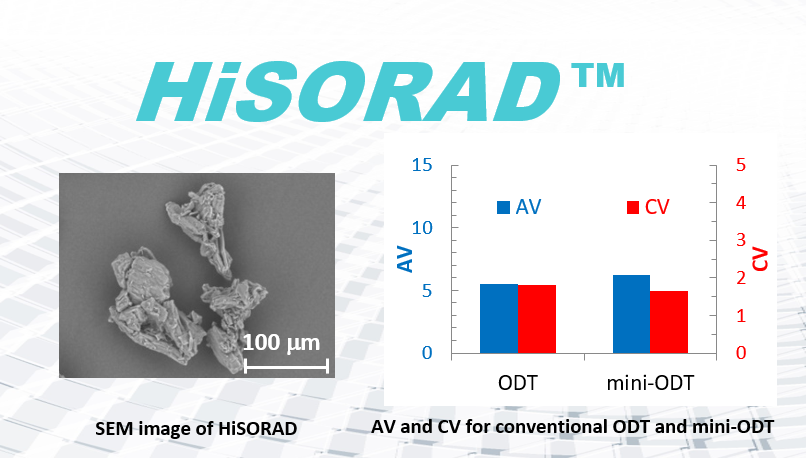
Direct compression is the simplest tableting method, contributing to both energy savings and cost advantages. However, maintaining content uniformity of active pharmaceutical ingredient (API) in tablets prepared by direct compression can be challenging, as powders with different physical properties are directly mixed without any pre-manufacturing process. HiSORAD is a state-of-the-art co-processed excipient for orally disintegrating tablet (ODT) produced by direct compression and has a non-spherical shape that may contribute to preventing segregation during the process (Fig.1). In this study, the content uniformity in ODT with HiSORAD was evaluated.1 In addition, mini-ODT was also evaluated since it is attracting attention as a pediatric formulation and seems to have more difficulty to maintain content uniformity in a tablet.
Application for conventional ODT
Content uniformity is often a challenge for low-dose tablets produced by direct compression. We chose 4% Enalapril Maleate (EM) as a model API. Its mean particle size was about 76 μm, being similar to that of HiSORAD (100 m). The tablet had enough performance as an ODT with practical tablet hardness and rapid disintegration being less than 30 sec. For the determination of content uniformity according to Ph. Eur 2. 9. 40, ten tablets of EM were randomly picked up and the acceptance value (AV) and coefficient variation (CV) were calculated. As a result, AV was less than 15 complying with the specification of Ph, Eur. Furthermore, CV was less than 2%.
Application for mini-ODT
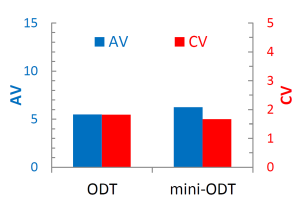
When 76 μm EM was contained in mini-ODT, AV and CV fell outside the specification. This is because the number of EM particles in a tablet is too low and the presence or absence of one EM particle in the tablet greatly contributes to the variation of content. Therefore, micronized EM with the mean particle size of 4 μm was introduced. In this case, AV and CV show similar values to conventional ODT. It was clear that pulverizing API and increasing the number of API particles in a tablet contributed to maintaining content uniformity. Furthermore, micronized particles assumed to stick to the porous surface of HiSORAD as Hejduk reported.2
In conclusion, HiSORAD is applicable not only to low-dose conventional ODT but also mini-ODT from a viewpoint of content uniformity. Especially for mini-ODT, it is important that the enough number of API particles is contained in a tablet and optimization of API size is required to maintain content uniformity.
Table 1 – Tablet condition:
| Tablet shape | Tablet weight | Mean particle size of EM | Component |
| Φ9 mm flat faced | 250 mg | 76 μm | EM (4%) + HiSORAD (95%) + sodium stearyl fumarate (SSF) (1%) |
| Φ2 mm biconvex | 6 mg | 4.0 μm | EM (4%) + HiSORAD (93%) + SSF (3%) |
Acknowledgements
This study was carried out under the collaborative research project with Heinrich Heine University. We would like to thank Dr. Kokott for useful discussions and providing data.
 Download the full article as a PDF here
Download the full article as a PDF here
References: 1) M. Kokott, et al., Eur. J. Pharm. Biopharm., 168, 122 (2021) 2) A. Hejduk, et al., Powder Technol., 387, 494 (2021)

