In vivo pharmacodynamics of lactoferrin-coupled lipid nanocarriers for lung carcinoma: intravenous versus powder for inhalation
Lung carcinoma characterized by high mortality rate and poor prognosis; the efficacy of drug delivery should improve drug exposure at the targeted site. This study aims at evaluating lactoferrin role as targeting ligand besides the administration route impact on tissue deposition and organ distribution. Lactoferrin (Lf)-coupled/uncoupled solid lipid nanoparticles (SLNs) were loaded with myricetin-phospholipid-complex (MYR-PH-CPX). Following physicochemical characterization, in-vitro antitumor activity and cellular uptake were investigated in A549-cell line. In-vivo deposition and biodistribution of fluorescently-labeled inhalable microparticles (with/without-Lf) were compared to intravenously administered fluorescently-labeled-SLNs (with/without-Lf) in mice.
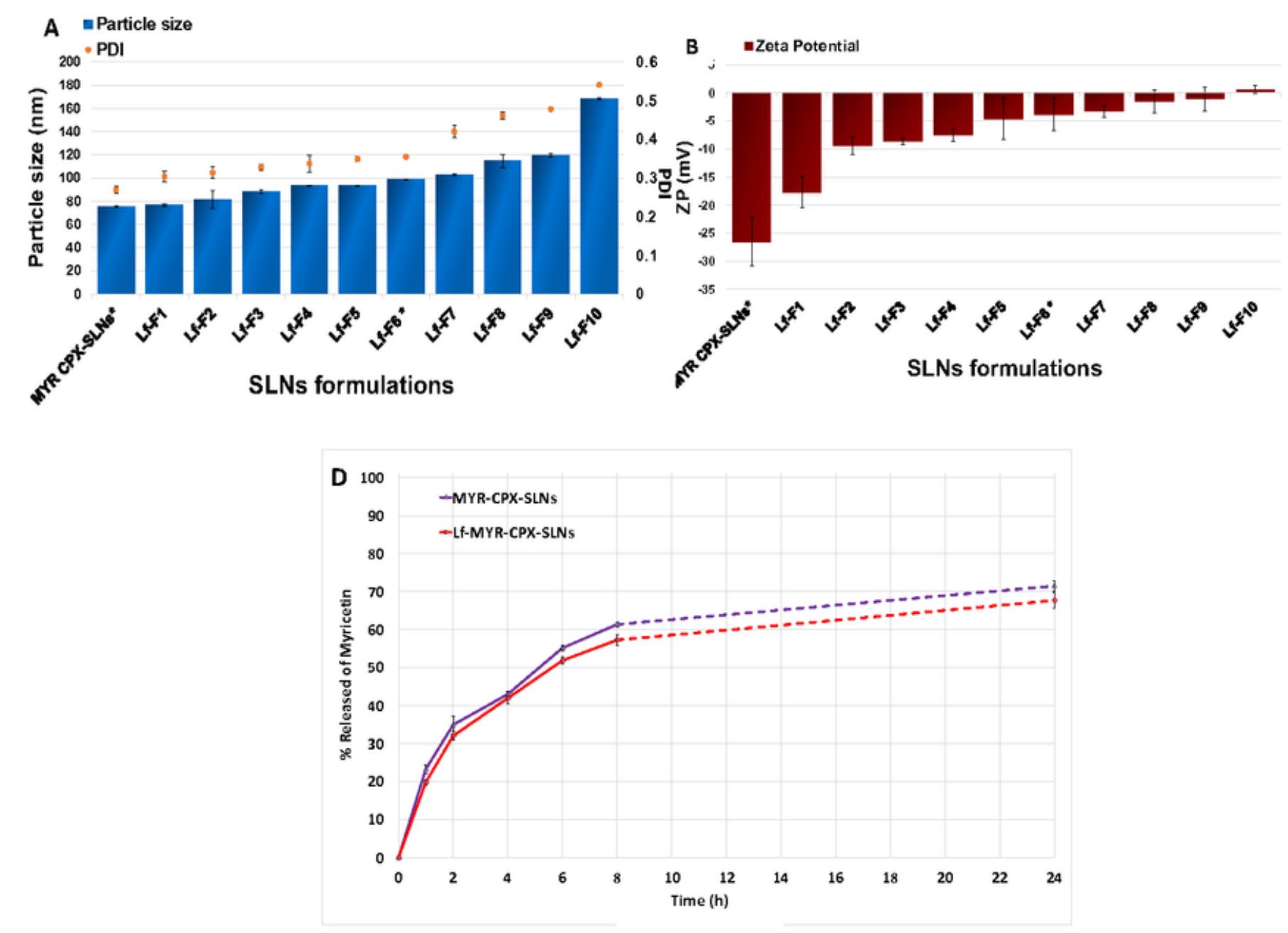
Lf-coupled-SLNs (98.59±0.47 nm), showed high entrapment efficiency (95.3±0.5%) and prolonged drug release. The in–vitro antitumor study showed reduction in IC50 for Lf-coupled-SLNs by ~2-and 3.5-fold relative to uncoupled-SLNs and MYR-PH-CPX, respectively confirming Lf role in enhancing antitumor activity by boosting cells internalization in shorter time. Furthermore, 3D-time laps confocal imaging showed that labeled-Lf-coupled-SLNs had a higher rate and extent of uptake in A549-cells compared to uncoupled-SLNs and free dye. In-vivo biodistribution proved that Lf enhanced pulmonary deposition of inhaled SLNs (~1.5 fold) and limited migration to the other organs within 6h relative to intravenous. Conclusively, local administration is superior due to less drug clearance resulting in lower toxicity accompanied by systemic application.
Download the full article as PDF here In vivo pharmacodynamics of lactoferrin-coupled lipid nanocarriers for lung carcinoma: intravenous versus powder for inhalation
or read more here
Materials
High purity myricetin was purchased from Shanghai Tauto Biotech Co. Ltd, Shanghai, China. Lipids like Gelucire 50/13 and Compritol 888 ATO, were gift samples from Gattefosse, Lyon, France. Soybean phosphatidylcholine (Lipoid® S 100) (purity > 96%) was purchased from Lipoid GmbH, Ludwigshafen, Germany. Coumarin-6 was acquired from Polysciences Europe GmbH, Hirschberg, Germany. Hoechst 33342, blue fluorescent stain specific for DNA (i.e., nuclei of eukaryotic cells), was received from Thermo Fisher Scientific, USA. Lactoferrin was kindly provided from Sigma Aldrich, Schnelldorf, Germany. O-phosphoric acid and Methanol (HPLC grade) were obtained from Merck, Massachusetts, USA. Lung epithelial cancer cell line A549 and Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from the American Type Culture Collection, ATCC, USA and Gibco, Basel, Switzerland, respectively. Sodium sulfite was purchased from El-Nasr Pharmaceutical Co., Cairo, Egypt.
In vivo pharmacodynamics of lactoferrin-coupled lipid nanocarriers for lung carcinoma: intravenous versus powder for inhalation, Dina M. Gaber, Noha Nafee, Ahmed O. Elzoghby, Maged W. Helmy, Osama Y. Abdallah, https://doi.org/10.21203/rs.3.rs-3334664/v1, This work is licensed under a CC BY 4.0 License.
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


