UV–VIS imaging-based investigation of API concentration fluctuation caused by the sticking behaviour of pharmaceutical powder blends
Abstract
Surface powder sticking in pharmaceutical mixing vessels poses a risk to the uniformity and quality of drug formulations. This study explores methods for evaluating the amount of pharmaceutical powder mixtures adhering to the metallic surfaces. Binary powder blends consisting of amlodipine and microcrystalline cellulose (MCC) were used to investigate the effect of the mixing order on the adherence to the vessel wall. Elevated API concentrations were measured on the wall and within the dislodged material from the surface, regardless of the mixing order of the components. UV imaging was used to determine particle size and the distribution of the API on the metallic surface. The results were compared to chemical maps obtained by Raman chemical imaging. The combination of UV and VIS imaging enabled the rapid acquisition of chemical maps, covering a substantially large area representative of the analysed sample. UV imaging was also applied in tablet inspection to detect tablets that fail to meet the content uniformity criteria. The results present powder adherence as a possible source of poor content uniformity, highlighting the need for 100% inspection of pharmaceutical products to ensure product quality and safety.
Introduction
Content uniformity is a quality measure of the final product required by regulatory bodies, such as the European Medicines Agency (EMA) and Food and Drug Administration (FDA), to ensure product quality, efficacy, and patient safety. Patient risk is particularly important for low-dose, highly potent drugs with narrow therapeutic windows, considering that even small variations in the active pharmaceutical ingredient (API) content can lead to ineffective doses or adverse effects (Goodwin et al., 2018, Muselík et al., 2014). The main sources of failed content uniformity are insufficient blend homogeneity, or further segregation of the initially well-mixed material during handling (Jakubowska and Ciepluch, 2021). Despite obtaining a uniform mixture, fluctuations in the API content may occur during various intermediate processes, including blending, transfer, storage, feeding, granulation, fluidization, die filling, compaction, and capsule filling (Garg et al., 2018). Furthermore, the properties of the final powder blend have a significant impact on critical quality attributes (CQA) of the final dosage form, such as dissolution profile, disintegration time, porosity, friability and hardness (Andrews, 2007).
The handling and processing of pharmaceutical materials are influenced by the sticking behaviour of the components. In the context of particle interactions, the terms sticking, agglomeration and adhesion are often used interchangeably to describe the attraction phenomenon between two solid bodies with a common contact surface (Petean and Aguiar, 2015, Zimon, 1982). The adhesion forces between particles and surfaces come as a result of electrostatic forces, capillary forces and van der Waals forces, among others (Beaudoin et al., 2015, Salazar-Banda et al., 2007). On the other hand, cohesion refers to the chemical forces holding similar particles together (Frabetti et al., 2021).
The quality of the final pharmaceutical product is influenced by the various factors that impact the adhesion and cohesion interactions of pharmaceutical powders. The surface roughness and the topography of the equipment directly affects the contact area between particles and the surface (Karner et al., 2014, Mazel et al., 2013, Wang et al., 2015). The sticking behaviour also depends on the material properties, such as the surface area, the polymorphic form and crystal morphology of the powder (Abdel-Hamid et al., 2011, Capece, 2019, Paul et al., 2020, Waknis et al., 2014). The adhesion of small particles on rough surfaces is primarily determined by the geometrical effects between the surface-particle system. In contrast, particles larger than the surface features have multiple contact areas, with both particle size and surface roughness significantly influencing adhesion (Katainen et al., 2006). Humidity causes the formation of liquid bridges and increases in the removal force, thereby altering the flow and adhesion behaviour of the material (Stevenson et al., 2023).
Previous studies have investigated the undesirable effect of powder adhesion during tableting and capsule filling. Punch sticking occurs when an API or excipient exhibits a stronger affinity for tablet punches (adhesion) than for the components of the formulation (cohesion), leading to tablet defects, manufacturing downtime, and yield losses (Chattoraj et al., 2018, Simmons, 2019, Simmons and Gierer, 2012, Takeuchi et al., 2020). Stickiness during the encapsulation process causes a large variability in capsule fill weight, and the repeated contact between the powder and metal parts may significantly affect machine performance (Podczeck, 2004). Even when utilizing excipients with satisfactory flow properties, such as microcrystalline cellulose (MCC), the phenomenon of powder sticking to the walls of the dosing nozzle has been observed (Patel and Podczeck, 1996, Tan and Newton, 1990). In the effort to mitigate adhesion to the tooling, lubricants are most often applied, however these may hinder drug dissolution (Abe and Otsuka, 2012, Uzunović and Vranić, 2007). Podzeck explored an alternative solution by modifying the stainless-steel surface of the tamping pins with various metal coatings (Podczeck, 1999).
Several experimental methods have been developed to detect and measure surface-powder interactions. The methods can be categorized into qualitative and quantitative techniques, each offering valuable insights into powder adhesion, thereby providing a more comprehensive understanding of the sticking phenomenon. Quantitative methods involve the direct measurement of the magnitude of powder adherence. In the centrifugal technique, particles deposited on the substrate detach during centrifugation. Image analysis is used to determine the number of adhered particles before and after centrifugation; the results are then analysed statistically to determine the average adhesion force (Booth and Newton, 1987, Petean and Aguiar, 2015, Thomas and Beaudoin, 2015). The development of the colloid-probe atomic force microscopy (AFM) allowed the investigation of surface forces between a single particle and the surface (Bunker et al., 2011, Ibrahim et al., 2000, Preedy et al., 2015). Imaging techniques have been previously used as qualitative measure of powder adhesion to compression tools. Scanning electron microscopy (SEM) and energy dispersive X‐ray spectroscopy (EDS) can visualize the material adhering to the tablet punches (McDermott et al., 2011, Neilly et al., 2009, Tsosie et al., 2017). Rhodes et al. utilized Raman chemical imaging to examine the material residue on the punch surface (Rhodes et al., 2022). Mollereau et al. used image analysis to detect and quantify the visual defects caused by tablet sticking (Mollereau et al., 2013). The aforementioned imaging techniques were primarily utilized for detecting tablet sticking; their suitability in the analysis of powder adherence during other pharmaceutical processes remains unexplored.
Recent advancements in machine vision technology provide a fast and cost-efficient approach for quality control and process monitoring in the pharmaceutical industry (Galata et al., 2021). In a study conducted by Wu et al., multispectral UV imaging was employed to visualize drug and excipient distribution in tablets containing glibenclamide, MCC and magnesium stearate (Wu et al., 2014). The versatility of this imaging technique has been demonstrated in various pharmaceutical applications, such as the surface analysis of multiple unit pellet systems (MUPS) (Novikova et al., 2016), determining tablet coating thickness (Novikova et al., 2017) and estimating tablet hardness and API content (Klukkert et al., 2016). Multispectral UV imaging was applied to detect tablets with inhomogeneous surface density (Klukkert et al., 2016). Mészáros et al. increased image acquisition speed by utilizing UV imaging exclusively in the 380–395 nm range. The technique was successfully used to determine API content and particle size from meloxicam tablets, a yellow model drug (Mészáros et al., 2022, Mészáros et al., 2020). However, these novel imaging methods have not yet been utilized to characterize powder adherence to the wall of manufacturing equipment. These imaging techniques can greatly contribute to the understanding of the sticking behaviour of pharmaceutical powders, the information gained this way can be used to prevent future inhomogeneity problems.
This article aims to explore methods for examining the sticking behaviour of pharmaceutical powder blends to stainless-steel surfaces during the homogenization process. The combination of quantitative methods and imaging techniques provides a more complex understanding of powder adherence. A comprehensive review of the relevant literature revealed that no results have been published up to now regarding the use of chemical imaging techniques to investigate powder adhesion to the surface of pharmaceutical equipment. Accordingly, the present work focused on the use of UV imaging to determine the distribution and particle size of the API particles. The feasibility of UV imaging to predict the tablet API content was also explored, contributing to the integration of machine vision into pharmaceutical quality control. The specific objectives include quantifying the API content in the powder adhering to the mixing vessel walls, assessing the importance of different mixing orders, and determining the API content in the material detached from the metallic surface. Rapid chemical imaging techniques such as UV–VIS imaging were employed to evaluate the distribution and particle size of the API on stainless-steel surfaces. To the authors knowledge, this is the first time that UV imaging has been used to characterize the adhesion of pharmaceutical particles to stainless-steel surfaces. Furthermore, we also intend to utilize UV imaging to detect tablets that deviate from the target API content.
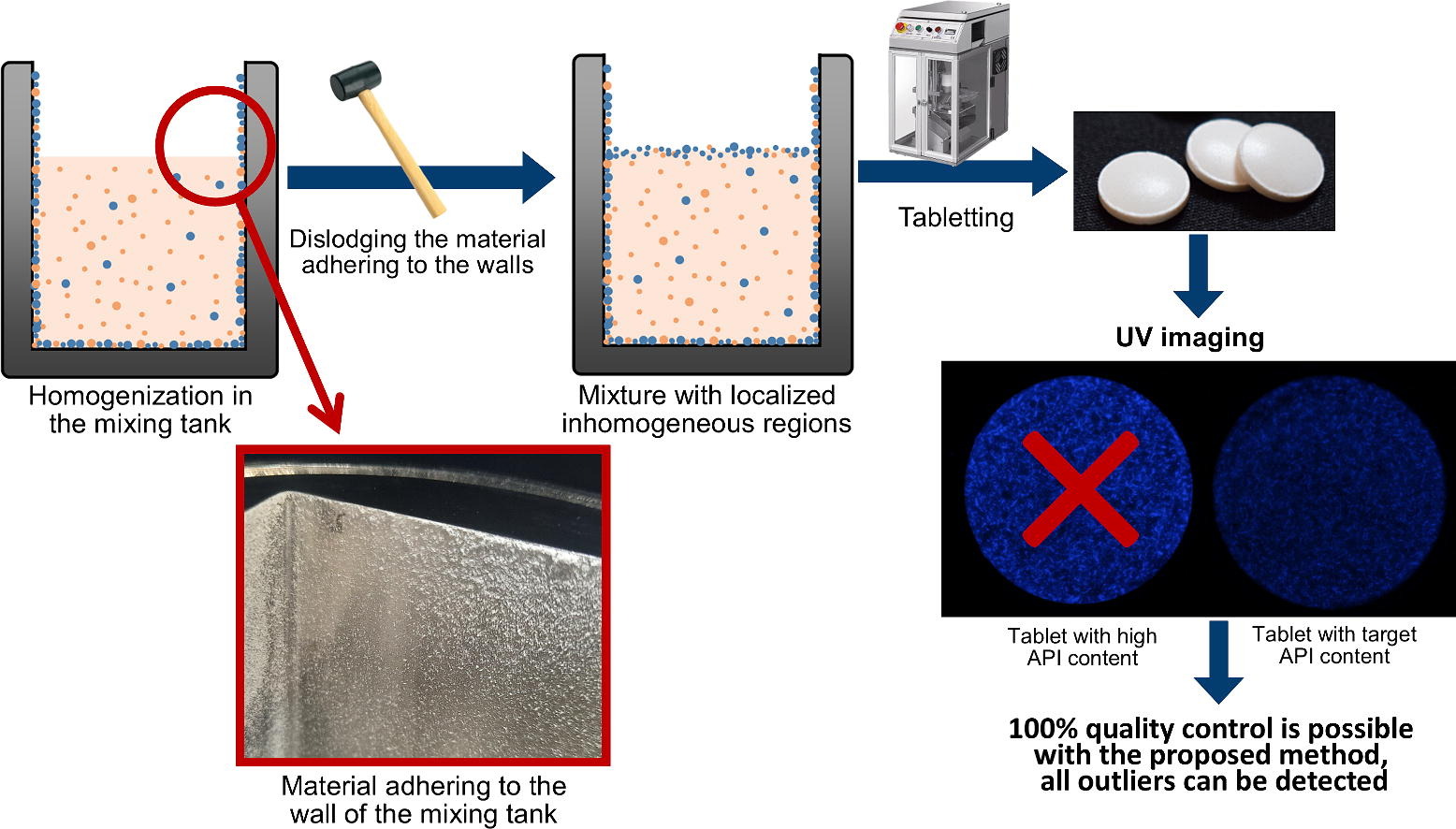
The binary powder mixture selected for this investigation contained amlodipine as model API and MCC as multipurpose excipient. Amlodipine is an antihypertensive and antiischemic drug with poor manufacturability, attributed to the sticking behaviour and unfavourable flow properties of the material. In contrast, MCC is a versatile pharmaceutical excipient used in tablets, capsules, pellets and granule formulations due to its excellent flow properties and good compressibility. Our aim was to explore methods for examining the sticking behaviour of pharmaceutical powder blends to stainless-steel surfaces during the homogenization process. The dislodgment of the material from the metallic surface due to a mechanical impact may lead to small volumes where API concentration deviates from the average value, potentially resulting in products with poor content uniformity. Detecting these outliers proves challenging with traditional analytical methods, emphasizing the importance of 100 % quality control during the manufacturing process.
Download the full article as PDF here: UV–VIS imaging-based investigation of API concentration fluctuation caused by the sticking behaviour of pharmaceutical powder blends
or read it here
Materials
Amlodipine besylate was purchased from Sigma-Aldrich. The API particles were ground in a mortar to obtain fine amlodipine. Microcrystalline cellulose (Vivapur 200) was obtained from JRS Pharma (Rosenberg, Germany).
Tablet Press:
100 mg of each sample obtained during powder homogenization described in section 2.2.1. (top, middle, bottom layer and dislodged material) were directly compressed into tablets at 5 kN compression force using a Dott Bonapace Cpr6 (Dott Bonapace, Limbiate, Italy) eccentric tablet press, equipped with an 8 mm flat punch. The tablets were analysed using Raman mapping and UV imaging.
Orsolya Péterfi, Lilla Alexandra Mészáros, Bence Szabó-Szőcs, Máté Ficzere, Emese Sipos, Attila Farkas, Dorián László Galata, Zsombor Kristóf Nagy, UV–VIS imaging-based investigation of API concentration fluctuation caused by the sticking behaviour of pharmaceutical powder blends, International Journal of Pharmaceutics, 2024, 124010, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124010.
Read also our introduction article on Microcrystalline Cellulose here:


