The development of a solid lipid nanoparticle (SLN)-based lacticin 3147 hydrogel for the treatment of wound infections
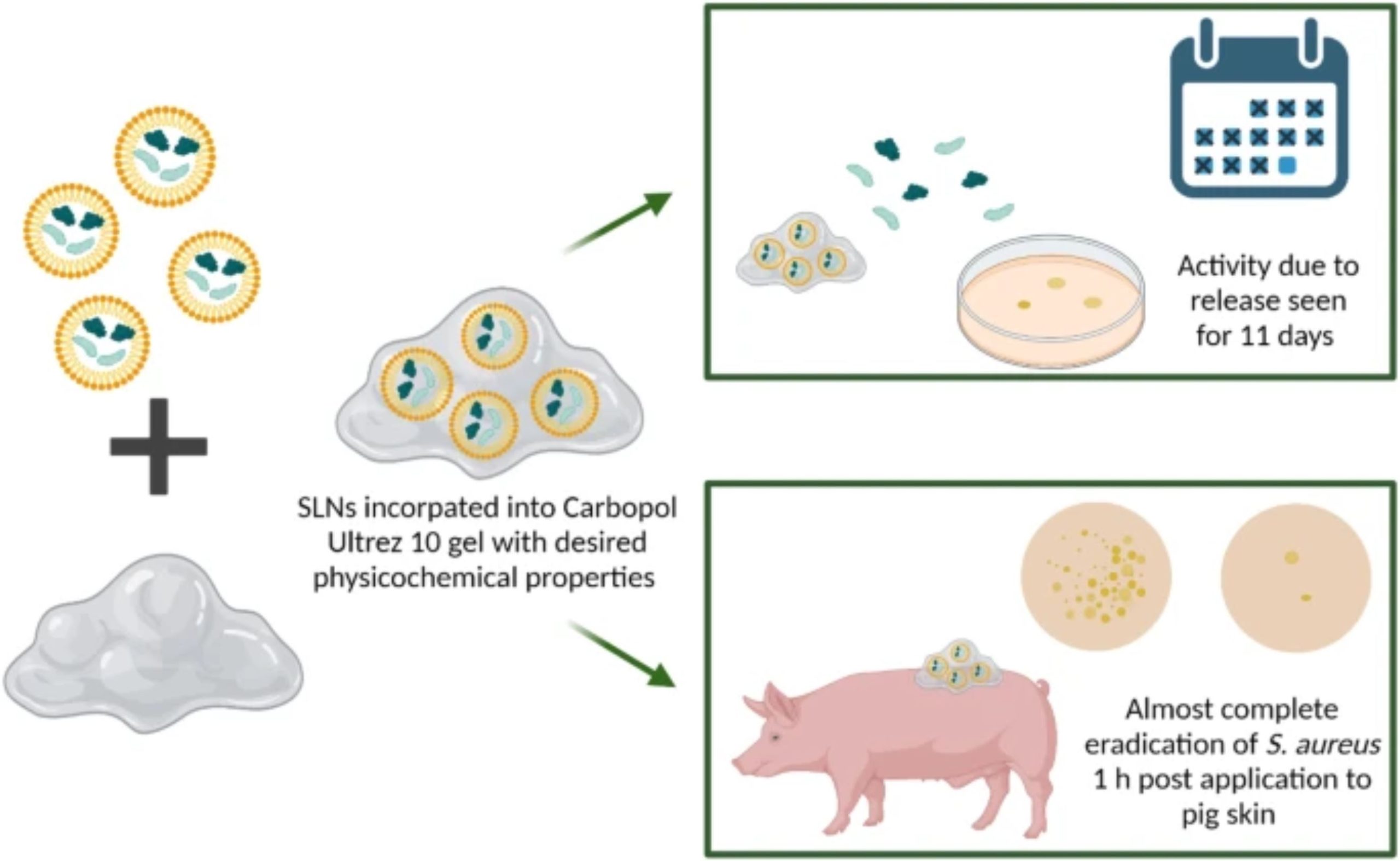
Chronic wounds affect millions of people globally. This number is set to rise with the increasing incidence of antimicrobial-resistant bacterial infections, such as methicillin-resistant Staphylococcus aureus (MRSA), which impair the healing of chronic wounds. Lacticin 3147 is a two-peptide chain bacteriocin produced by Lactococcus lactis that is active against S. aureus including MRSA strains. Previously, poor physicochemical properties of the peptides were overcome by the encapsulation of lacticin 3147 into solid lipid nanoparticles. Here, a lacticin 3147 solid lipid nanoparticle gel is proposed as a topical treatment for S. aureus and MRSA wound infections. Initially, lacticin 3147’s antimicrobial activity against S. aureus was determined before encapsulation into solid lipid nanoparticles.
An optimised gel formulation with the desired physicochemical properties for topical application was developed, and the lacticin-loaded solid lipid nanoparticles and free lacticin 3147 aqueous solution were incorporated into separate gels. The release of lacticin 3147 from both the solid lipid nanoparticle and free lacticin gels was measured where the solid lipid nanoparticle gel exhibited increased activity for a longer period (11 days) compared to the free lacticin gel (9 days). Both gels displayed potent activity ex vivo against S. aureus-infected pig skin with significant bacterial eradication (> 75%) after 1 h. Thus, a long-acting potent lacticin 3147 solid lipid nanoparticle gel with the required physicochemical properties for topical delivery of lacticin 3147 to the skin for the potential treatment of S. aureus-infected chronic wounds was developed.
Download the full article as PDF here The development of a solid lipid nanoparticle (SLN)-based lacticin 3147 hydrogel for the treatment of wound infections
or read it here
Materials
L. actis DPC6577, a lacticin 3147-producing strain, and pig skin were provided by Teagasc Food Research Centre Moorepark, Cork, Ireland. Listeria monocytogenes culture (ATCC 1916) was purchased from ATCC. S. aureus DSMZ20231 was purchased from DSMZ, the German collection of microorganisms and cell cultures. S. aureus Xen-29 was supplied by our collaborators in APC Microbiome Ireland, Cork. Brain heart infusion (BHI) broth, BHI agar, bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), ethanol (EtOH), hydroxypropyl methylcellulose (HPMC with a molecular weight of 5.5 kDa), glutaraldehyde, phosphate-buffered saline (PBS), sodium sulphate, triethanolamine (TEA), tryptic soy agar (TSA) and tryptic soy broth (TSB) were purchased from Sigma-Aldrich Ireland. Isopropanol (IPA, > 99.9%) and yeast extract were purchased from Fisher Scientific Ireland Ltd. Fasted state simulated intestinal fluid (FaSSIF) was purchased from Biorelevant.com Ltd., UK. Transcutol P was gifted from Gattefossé, France, Softisan 601 was gifted from 101 Oleochemical Pharma, Germany, and Kolliphor RH40 was received as a gift from BASF, Germany. Carbopol® Ultrez 10 (Ultrez 10) was gifted from The Lubrizol Corporation, USA. Deionised (DI) water was obtained from the Elga PURELAB system.
Ryan, A., Patel, P., Ratrey, P. et al. The development of a solid lipid nanoparticle (SLN)-based lacticin 3147 hydrogel for the treatment of wound infections. Drug Deliv. and Transl. Res. (2023). https://doi.org/10.1007/s13346-023-01332-9

