Development of Lipid-based SEDDS Using Digestion Products of Long-chain Triglyceride for High Drug Solubility: Formulation and Dispersion Testing
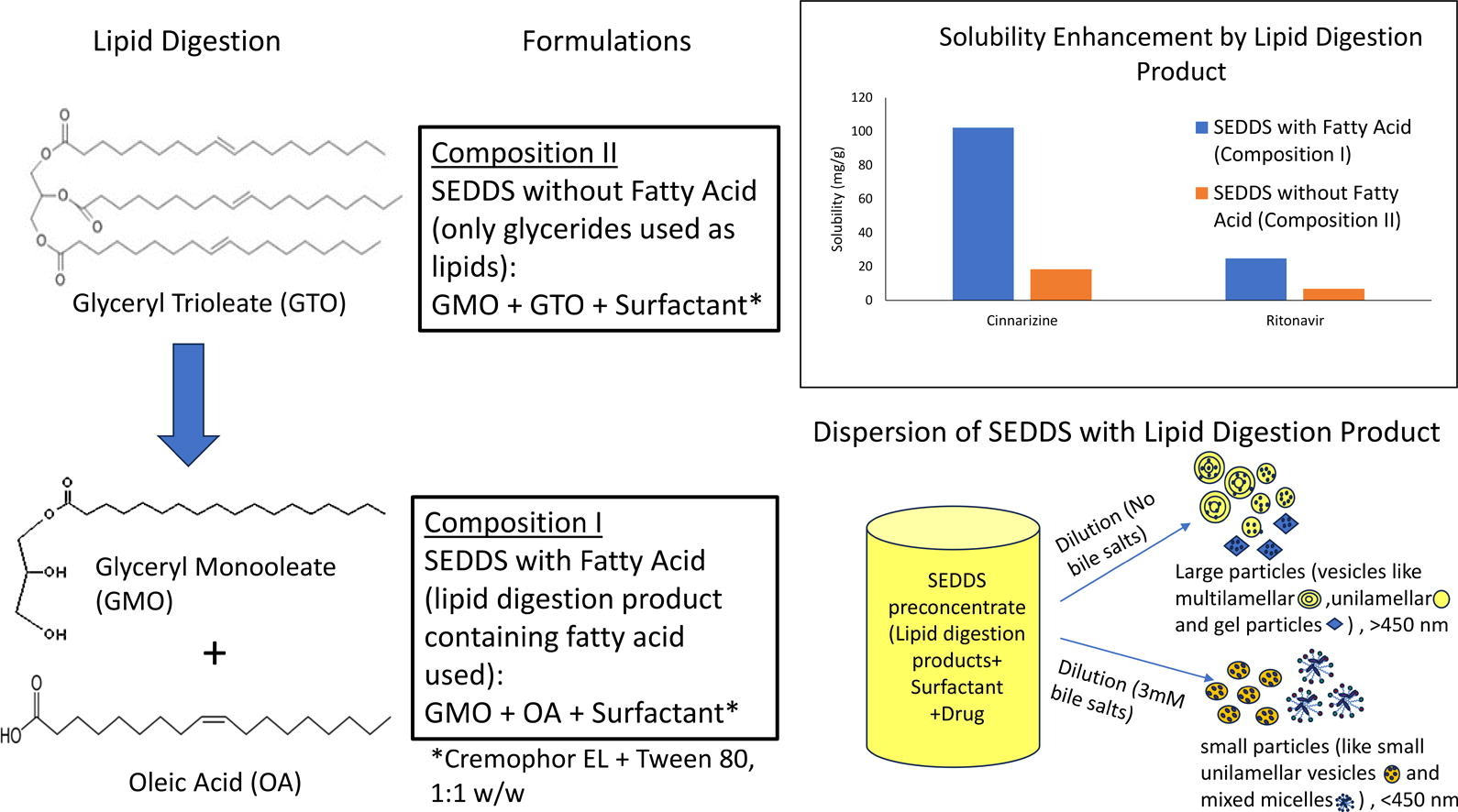
A self-emulsifying drug delivery system (SEDDS) containing long chain lipid digestion products (LDP) and surfactants was developed to increase solubility of two model weakly basic drugs, cinnarizine and ritonavir, in the formulation. A 1:1.2 w/w mixture of glyceryl monooleate (Capmul GMO-50; Abitec) and oleic acid was used as the digestion product, and a 1:1 w/w mixture of Tween 80 and Cremophor EL was the surfactant used. The ratio between LDP and surfactant was 1:1 w/w.
Since the commercially available Capmul GMO-50 is not pure monoglyceride and contained di-and-triglycerides, the digestion product used would provide 1:2 stoichiometric molar ratio of monoglyceride and fatty acid after complete digestion in gastrointestinal fluid. Both cinnarizine and ritonavir had much higher solubility in oleic acid (536 and 72 mg/g, respectively) than that in glyceryl monooleate and glyceryl trioleate. Therefore, by incorporating oleic acid in place of glyceryl trioleate in the formulation, the solubility of cinnarizine and ritonavir could be increased by 5-fold and 3.5-fold, respectively, as compared to a formulation without the fatty acid.
The formulation dispersed readily in aqueous media, and adding 3mM sodium taurocholate, which is generally present in GI fluid, remarkably improved the dispersibility of SEDDS and reduced particle size of dispersions. Thus, the use of digestion products of long-chain triglycerides as components of a SEDDS system can enhance the drug loading of weakly basic compounds and increase dispersibility in GI fluids.
Read more here
Materials
Surfactants (Cremophor EL and Tween 80) and long-chain lipid digestion products (oleic acid and glyceryl monooleate) used in the present investigation are listed in Table 1 along with their trade names, manufacturers, chemical structures, and compositions. All data in the table are taken from the manufacturers’ brochures. It may be noted that most of these materials are not chemically pure species, and rather they exist as mixtures with other related materials.
Heta H. Desai, Abu T. M. Serajuddin, Development of Lipid-based SEDDS Using Digestion Products of Long-chain Triglyceride for High Drug Solubility: Formulation and Dispersion Testing, International Journal of Pharmaceutics,
2024, 123953, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123953.
Read also more on our introduction article on Parental Excipients here:


