Nose to brain delivery of melatonin lipidic nanocapsules as a promising post-ischemic neuroprotective therapeutic modality
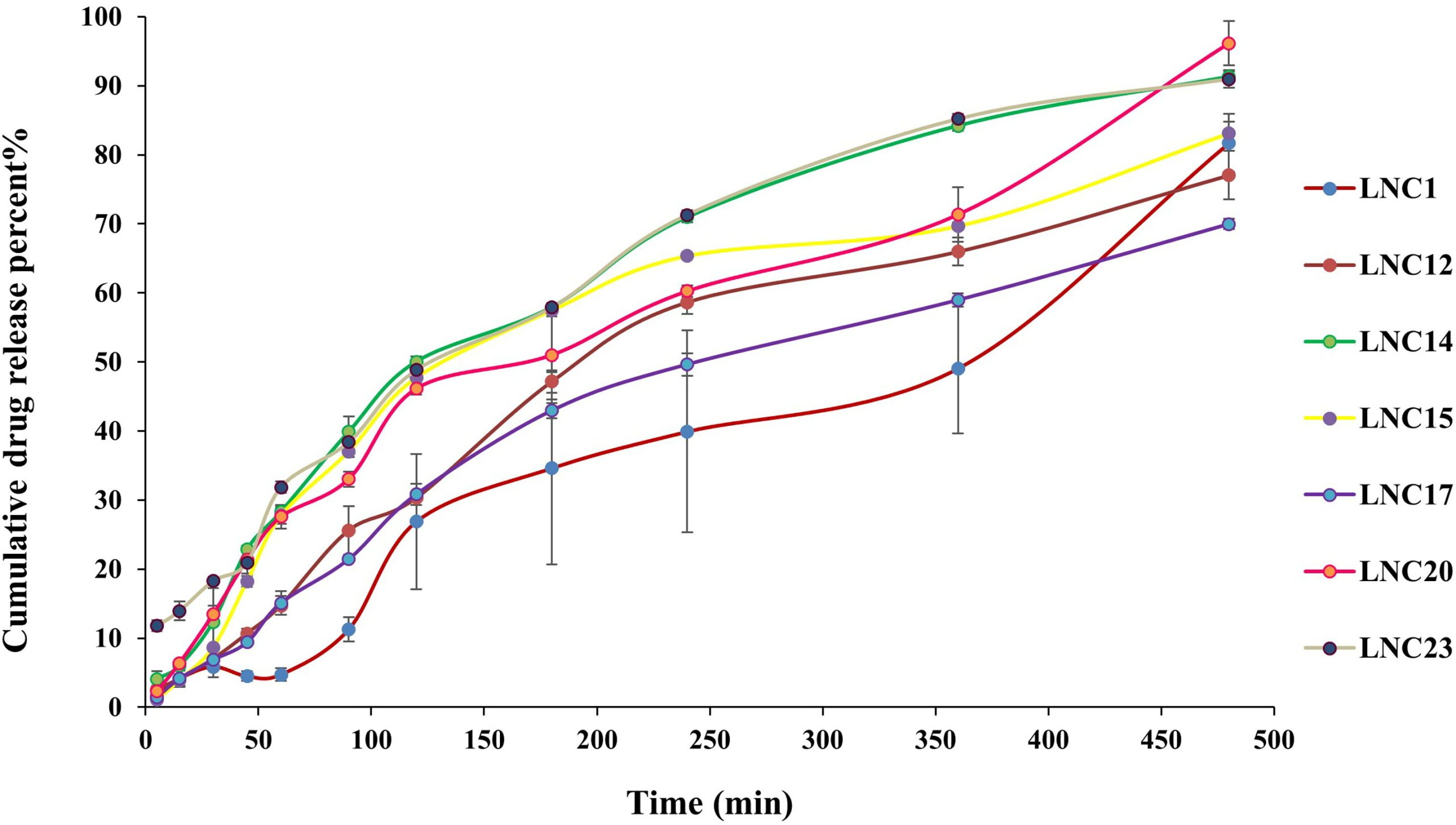
Ischemic stroke accounts for about 87% of all strokes, causing long-term disability in adults, and is the second leading cause of death worldwide. In search of new therapeutic modalities, the use of neuroprotective agents loaded in nanocarriers to be delivered by noninvasive means (i.e. via intranasal route) became a popular approach. In the current study, melatonin (MEL) was loaded in lipidic nanocapsules (LNCs) prepared using the phase inversion method, and characterized in terms of size, polydispersity, zeta potential, in vitro drug release, viscosity, storage stability, and ex vivo permeation across sheep nasal mucosa. Moreover, MEL-LNCs were tested for efficacy in cerebral ischemia/reperfusion (I/R/) injury model through histopathological assessment, and analysis of oxidative stress markers, pro-inflammatory cytokines, and apoptotic markers. Results showed that LNCs exhibited particle size ranging from 18.26 to 109.8 nm, negative zeta potential, good storage stability, spherical morphology, and a burst release followed by a sustained release pattern. LNCs exhibited 10.35 folds higher permeation of MEL than the drug solution across sheep nasal mucosa. Post-ischemic intranasal administration of MEL-LNCs revealed lowering of oxidative stress manifested by a decrease in malondialdehyde levels, and elevation of glutathione and superoxide dismutase levels, lowering of the inflammatory markers tumor necrosis factor-α, NO, myeloperoxidase, and significant inhibition of Caspase-3 activity as an apoptotic marker. Western blot analysis delineated a recovery of protein expression Nrf-2 and HO-1 with downregulation in the parent inflammatory markers nuclear factor kappa B p65, inducible nitric oxide synthase, Bax, and Cytochrome C expressions, and upregulation of B-cell lymphoma-2 Bcl-2, hence promoting neuronal survival. This was supported by histological evidence, revealing significant restoration of hippocampal neurons. In light of the above, it can be concluded that MEL-LNCs could be a promising delivery system for nose to brain delivery for treatment of cerebral ischemia.
Materials
MEL was purchased from skin actives scientific, USA. Solutol HS15, acetonitrile, and water (HPLC grade) were purchased from Sigma-Aldrich Co., Germany, Labrafil M1944 CS was kindly provided as a gift from Gattefosse Co., France. Epikuron 200 (Soya bean lecithin) was kindly provided as a gift from Cargill Co., Germany. Thiopental was purchased from Sigma-Tec Pharmaceutical Ind., Egypt.
Download the research paper as PDF: Nose to brain delivery of melatonin lipidic nanocapsules as a promising post-ischemic neuroprotective therapeutic modality
or read more
(2022) Nose to brain delivery of melatonin lipidic nanocapsules as a promising post-ischemic neuroprotective therapeutic modality, Drug Delivery, 29:1, 2469-2480, DOI: 10.1080/10717544.2022.2104405

