Liqui-Tablet: the Innovative Oral Dosage Form Using the Newly Developed Liqui-Mass Technology
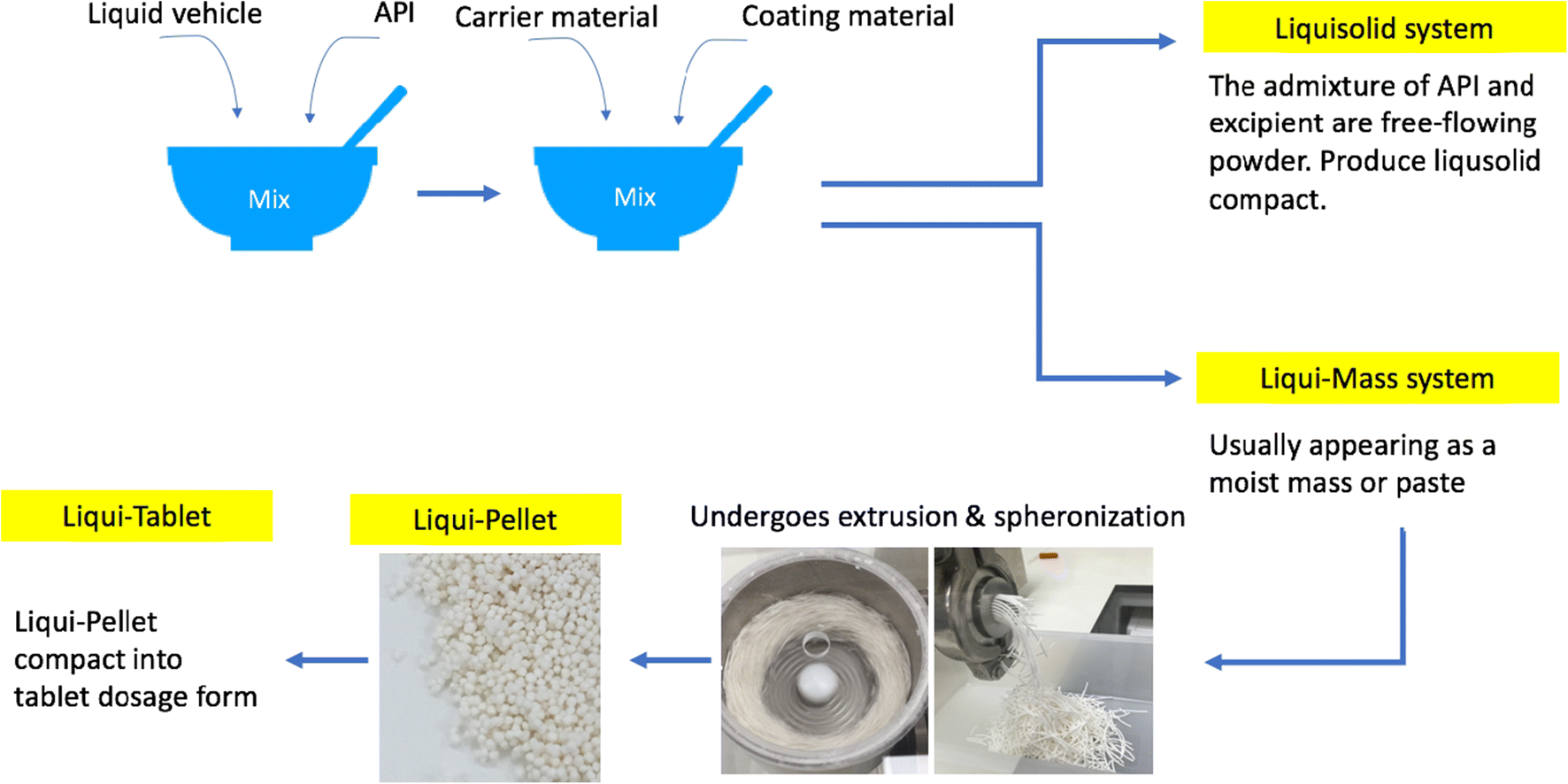
In this study, an attempt was made to produce Liqui-Tablets for the first time. This was carried out through the compaction of naproxen Liqui-Pellets. The incentive to convert the novel Liqui-Pellet into Liqui-Tablet was due to the array of inherent advantages of the popular and preferred tablet dosage form. The study showed that naproxen Liqui-Tablet could be successfully produced and the rapid drug release rate (100% drug release ~ 20 min) could be achieved under pH 1.2, where naproxen is insoluble. It was observed that the different pH of the dissolution medium affected the trend of drug release from formulations with varying amounts of liquid vehicle. The order of the fastest drug-releasing formulations was different depending on the pH used. The presence of Neusilin US2 showed considerable enhancement in the drug release rate as well as improving Liqui-Tablet robustness and hardness. Furthermore, images from X-ray micro-tomography displayed a uniform distribution of components in the Liqui-Tablet. The accelerated stability studies showed acceptable stability in terms of dissolution profile.
Download the full article here: Liqui-Tablet – The Innovative Oral Dosage Form Using the Newly Developed Liqui-Mass Technology
or continue reading here: Lam, M., Asare-Addo, K. & Nokhodchi, A. Liqui-Tablet: the Innovative Oral Dosage Form Using the Newly Developed Liqui-Mass Technology. AAPS PharmSciTech 22, 85 (2021). https://doi.org/10.1208/s12249-021-01943-w
Materials
Materials included naproxen (TCI, Japan), Avicel PH 101 (FMC corp., UK), Aerosil 300 (Evonik Industries AG, Hanau, Germany), Primojel (DFE Pharma, Goch, Germany), Neusilin US2 (Fuji Chemicals, Japan), sodium bicarbonate, (Acros, NJ, USA), and Tween 80 (Acros, Netherlands). All other reagents and solvent were of analytical grades.
Conclusion
The Liqui-Tablet formulation was successfully manufactured for the first time using naproxen as the drug model. In comparison to the rapid drug release of naproxen Liqui-Pellet from previous studies, it can be concluded that the rapid drug release is maintained in Liqui-Tablet dosage form at acidic pH. Nonetheless, when comparing the dissolution profile of the best naproxen Liqui-Tablet with other studies concerning naproxen solid dispersion and liquisolid compact, the drug release rate of Liqui-Tablet is more rapid. It is interesting how the liquid vehicle in naproxen Liqui-Tablet under pH 7.4 actually slows down the drug release rate. This is possibly due to liquid vehicle binding effect within the Liqui-Tablet, which reduces the propensity for disintegration. The disintegration step seems to be the rate-determining step at this pH as API solubility is no longer an issue. Compression force show little influence on Liqui-Tablet hardness; however, liquid vehicle concentration has a considerable impact on Liqui-Tablet hardness.

