Liquid API Feeding in Pharmaceutical HME: Novel Options in Solid Dosage Manufacturing
Hot melt extrusion (HME) is a common unit operation. It is broadly applicable in the pharmaceutical industry and can be implemented in a continuous manufacturing line. However, the conventional way of active pharmaceutical ingredient (API) feeding with a pre-blend consisting of a powdered API and a polymer does not allow the flexibility and agility to adjust the process parameters, which is generally an essential part of continuous manufacturing. In addition, this method of API feeding may result in the segregation of the individual powder components or agglomeration of highly cohesive materials, leading to an inhomogeneous API content in the extrudates, especially at low doses. In this study, the universal applicability of liquid side feeding in pharmaceutical HME was demonstrated using various APIs suspended or dissolved in water and fed as suspension or undersaturated, supersaturated, and highly concentrated solutions into anterior parts of the extruder. The extrudates were characterized in terms of their API content, residual moisture content, and solid-state of the API embedded in the polymer. The results show that a uniform API content without major deviations can be obtained via this method. Furthermore, the residual moisture content of the extrudates was low enough to have no significant influence on further processing of the final dosage form. In summary, this advanced way of feeding allows an accurate, flexible, and agile feeding of APIs, facilitating the production of personalized final dosage forms and a novel option to link the manufacturing of the drug substance and the drug product.
Introduction
The manufacturing of pharmaceutical dosage forms has been gradually transitioning from the batch mode to continuous manufacturing. In continuous manufacturing, pharmaceutical materials flow through an end-to-end manufacturing line without the necessity of interruption. In contrast, batch manufacturing involves discontinuous manufacturing, which means executing each processing step separately (Burcham et al., 2018, Helal et al., 2019, Vanhoorne and Vervaet, 2020). After each processing step, the individual batches are analyzed in terms of their critical quality attributes (CQAs) and are either released for further processing steps or discarded. Since individual batches are generally produced on large scale, a major loss of material can occur if the batch acceptance limits are not met (Schaber et al., 2011). Having to discharge significant amounts of material can be prevented by performing active quality control via real-time monitoring, also known as process analytical technology (PAT) implemented in a continuous manufacturing line. Thereby, only those amounts of intermediates and products, that do not comply with the CQAs rather than complete batches are discarded automatically (Fonteyne et al., 2015, Helal et al., 2019).
In addition, continuous manufacturing enables real-time feedback control over the process. This means that the process parameters can be individually adjusted during the running process to achieve the specified CQAs, and discarding the inappropriate material is required (Sacher et al., 2020a). This flexible and agile adjustment of process parameters also enables the manufacturing of personalized dosage forms specially adapted to the patient’s needs (Badman et al., 2019).
Hot melt extrusion (HME) is an example of a continuously operated unit operation in pharmaceutical manufacturing. In the last decades, HME has gained importance due to its wide range of applications, including the production of implants, films, (micro)pellets, and tablets (Koutsamanis et al., 2023, Patil et al., 2016, Repka et al., 2007, Spoerk et al., 2022). In addition, HME can provide beneficial product properties, e.g., including taste masking, sustained release, and enhanced solubility of active pharmaceutical ingredients (APIs) (Maniruzzaman et al., 2012, Maniruzzaman et al., 2013, Vithani et al., 2013). The latter is caused by the formation of amorphous solid dispersions (ASDs) due to molecular mixing of a polymeric material and an API. This not only increases the bioavailability and the duration of the drug action in the body but also reduces side effects (Breitenbach, 2002, Repka et al., 2018).
Conventionally, in the production of ASDs, the polymer and other meltable functional excipients are pre-blended with the API and fed via a feeder (Eggenreich et al., 2017), as shown in Figure 1. The advantages of HME have been proven via this way of API feeding, but it does not allow a flexible and agile adjustment of the API content in the pre-blend and the extrudates, which makes this approach unsuitable for the manufacturing of personalized dosage forms (Lee et al., 2015). In addition, for cohesive materials and especially at low doses, this process mode can create inhomogeneities in the pre-blend due to demixing and segregation of the individual components or agglomerates of highly cohesive materials and thus, pose problems with accurate dosing of the API into the extruder (Jakubowska and Ciepluch, 2021), where alternative approaches were shown to be necessary (Sacher et al., 2020b).
For conventional feeding via pre-blending, the availability of the API as a solid powder is essential, and the particle size distributions (PSDs) of API and polymer should be similar in order to minimize segregation and other undesired effects. However, producing a dry API powder (primary manufacturing) is an elaborate process, that includes crystallization, filtration, washing, and drying. Especially the drying step can reduce the API quality dramatically, as particle properties are often affected (Papadakis and Bahu, 1992). Liquid feeding during secondary manufacturing with dissolved or suspended API allows to skip the primary drying step while maintaining the API quality. It opens new options to link primary and secondary manufacturing, and enables local end-to-end production sites. As a result, the dependence on API supply from overseas can be reduced. This approach could help to reduce the vulnerability of supply chains, which became obvious during recent crises like the pandemic (Chapman et al., 2022).
The liquid feeding technology was used by Llusa et al. (2016) to achieve homogeneous low-dose feeding. The powdered API was dissolved and fed as a liquid solution in the first extruder sections. Extrudates with a homogeneous ibuprofen content of below 0.05wt.% in the strands were prepared. Chamberlain et al. (2022) used a solution with a pramipexole content of 4 wt.% to produce extrudates with a target API content of 0.1 wt.%. Baumgartner et al. (2016) used side-feeding to embed an aqueous nano-suspension into different polymeric matrices. Liquid feeding in pharmaceutical HME supports a more flexible and agile adjustment of the process and product. This allows individual manufacturing of extrudates with varying API concentrations and, ultimately, the production of personalized dosage forms. Moreover, liquid feed rates can be precisely controlled.
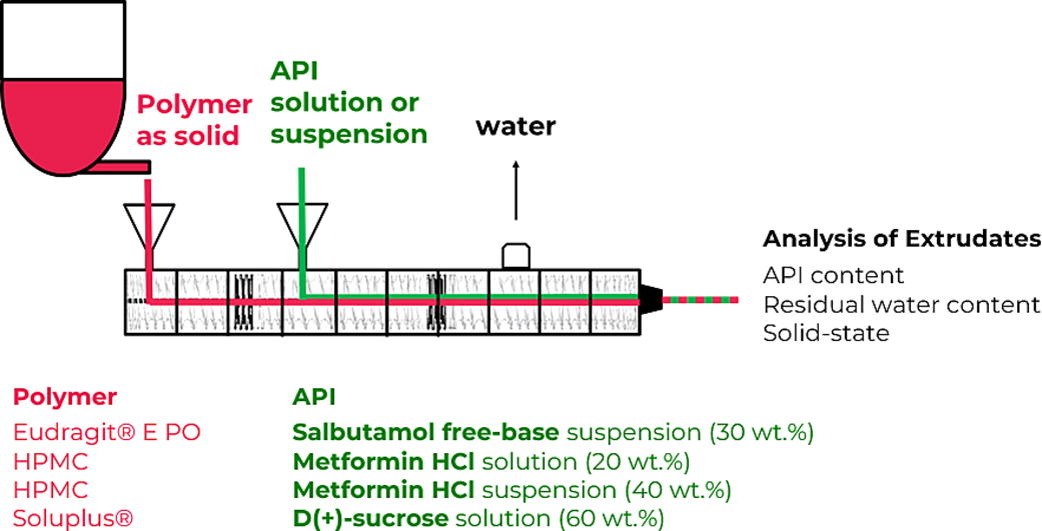
The aim of this study is to demonstrate the feasibility of liquid feeding into HME as a novel process link between the manufacturing of the drug substance and the drug product. To test the broad applicability, various combinations of APIs and polymers were assessed. Several model APIs with different physical properties were examined, including poorly water-soluble APIs (i.e., salbutamol free-base) and readily water-soluble APIs (i.e., metformin HCl and D(+)-sucrose). The APIs were fed as a suspension (salbutamol free-base), undersaturated and supersaturated solution (metformin HCl), and highly concentrated solution (D(+)-sucrose). In each case, water was used as solvent and each API was embedded in various polymers, including Eudragit® E PO, hydroxypropylmethylcellulose (HPMC) and Soluplus®. The extruded strands were analyzed in terms of their moisture content, embedded API solid-state and uniformity of the API content.
Materials
In this study, three model APIs were embedded in various polymers as a matrix. Salbutamol free-base (Shenzhen Nexconn Pharmatechs Ltd, China) was incorporated in Eudragit® E PO (Evonik Industries AG, Germany). Metformin HCl (Merck KGaA, Germany) was embedded in an HPMC (Thermo Fisher Scientific Inc., USA) matrix. D(+)-sucrose (Carl Roth GmbH + co. KG, Germany) was embedded in Soluplus® (BASF SE, Germany). These combinations are hereinafter referred to as ‘Salbutamol/Eudragit’, ‘Metformin/HPMC’
Read more on Liquid API Feeding in Pharmaceutical HME
Lisa Kuchler, Martin Spoerk, Simone Eder, Aygün Doğan, Johannes Khinast, Stephan Sacher, Liquid API Feeding in Pharmaceutical HME: Novel Options in Solid Dosage Manufacturing, International Journal of Pharmaceutics, 2023, 123690, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.123690.

