Lubricant Sensitivity of Direct Compression Grades of Lactose in Continuous and Batch Tableting Process
Abstract
Introduction
Modern pharmaceutical manufacturing is revolutionising production processes in the pharmaceutical industry, with a focus on continuous processes [1,2]. This shift has forced the industry to redesign batch-wise processing into integrated continuous processing. Continuous processes offer significant advantages in producing tablets, as they enhance throughput and increase the robustness of production. Additionally, process control tools, such as process analytical technology (PAT), enable the detailed monitoring of processes [3,4,5].
Tablets are one of the most common pharmaceutical dosage forms, and their production requires the use of many ingredients. In addition to active pharmaceutical ingredients (APIs), excipients are used to facilitate the tableting process. Fillers enable tablet formation, and lubricants facilitate the tableting process. To obtain a blend suitable for compression from a process perspective, APIs are mixed with appropriate excipients that provide essential features, such as acceptable flowability and adequate compressibility.
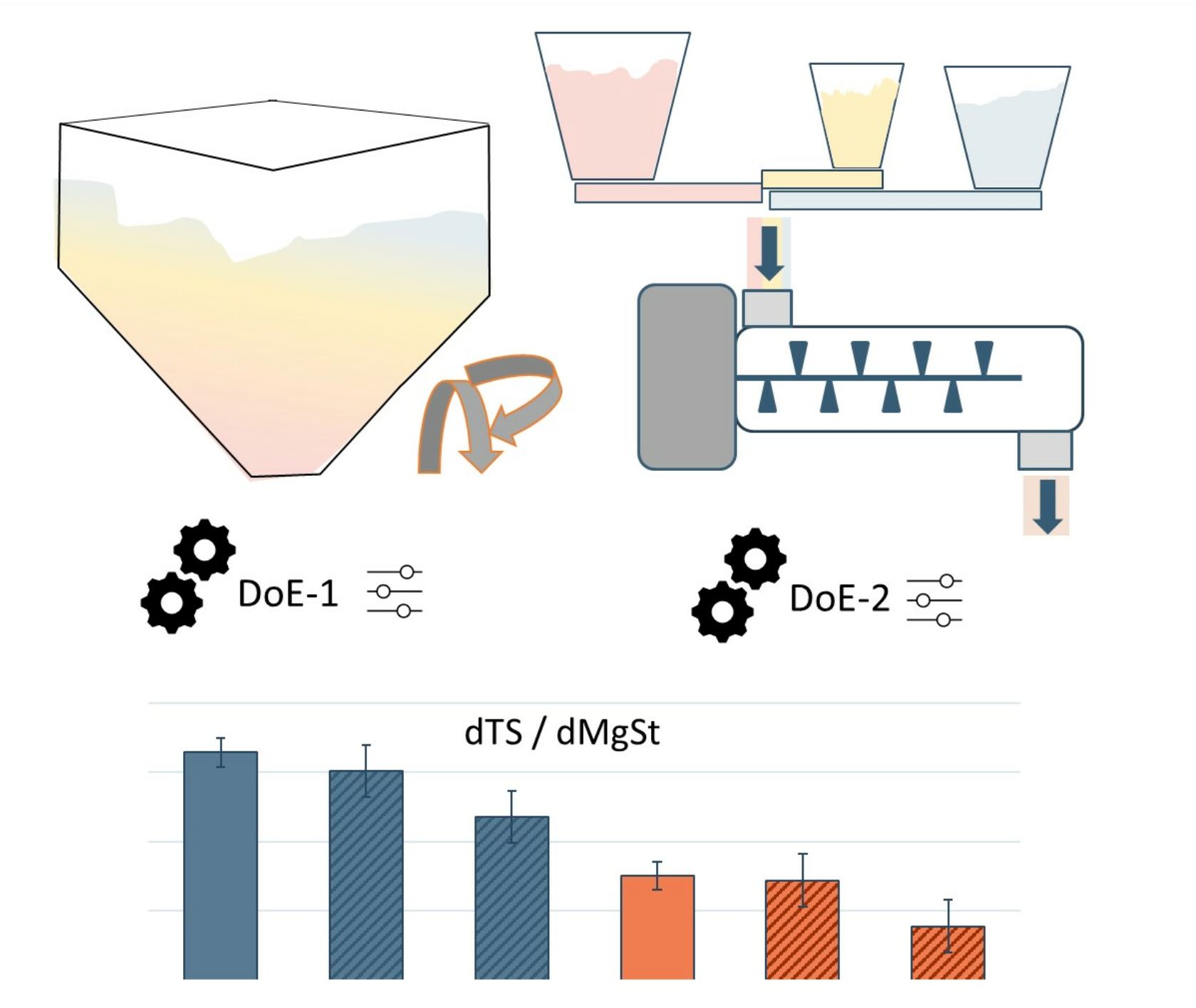
Traditionally, pharmaceutical powder blending is performed using batch technology. Batch blending involves loading powders into large vessels (e.g., bins) that are tumbled for a fixed number of revolutions before the material is discharged and processed further. Although this technology has been extensively studied and explored, it presents some criticalities. Batch blending provides difficulty in scaling up, limited flexibility in batch sizes, and challenges for batch reproducibility. One of the major reasons for selecting continuous processes is to address these issues.
Continuous processes might, however, be difficult to design, as the existing knowledge on batch processes is not directly transferable to continuous processes. In order to transform batch production into continuous production, different manufacturing steps need to be re-designed. Multiple studies have compared the effect of raw material properties in batch and continuous processes. The research on continuous processes is increasing annually, focusing on the effects of process parameters, tablet characteristics, and formulation choices [6,7,8,9]. Additionally, research has been focused on the role of raw materials in different operating units, like feeders [10,11,12,13,14] and blenders [15,16,17,18,19,20,21,22]. Several studies have demonstrated that it is challenging to homogeneously mix materials with major differences in the particle size distribution (PSD) in standard batch processes, potentially causing segregation [23]. Since the scale of blending and the residence time in continuous manufacturing is significantly shortened, other studies have shown that this has a positive effect on blend homogeneity. So far, no studies have been identified that compare the impact of material properties in batch and continuous blending processes on the risks of over-lubrication.
When blending in a continuous direct compression (CDC) line, there could be a risk of over-lubricating the formulation. Excipients play a vital role in mitigating this risk. Excipients are required to be consistent; they need to have a proper powder flow to produce stable blends with other ingredients, and they should be compactable [24]. The correct use of lubricants in formulations is challenging, particularly for the development of formulations suitable for a CDC. Lubricants are required to eject tablets from dies without defects, but the inter-particle bonds lose strength owing to the potential formation of a thin layer of lubricant that covers APIs and excipient particles.
In this study, we investigated the role of anhydrous lactose and granulated lactose in both continuous and batch processes. These two types of lactose are ideal for both batch and continuous manufacturing because of their excellent powder flow and compactibility. Granulated lactose is composed of α-lactose monohydrate, whereas anhydrous lactose mainly consists of anhydrous β-lactose (80% w/w), with the remainder being anhydrous α-lactose [25,26,27,28]. Therefore, it is expected that these materials will exhibit different sensitivities to changes in the process settings for both technologies. Batch blending was performed using a tumble blender, whose design is characterised by an optimised geometry that ensures high mixing efficiency with no dead zones. Continuous blending was performed by a horizontal blender. The powder ingredients were continuously dosed directly into the blender by means of three different loss-in-weight feeders (LIW).
A process can become robust as soon as the correlations between critical powder attributes and the critical parameters of the process are well understood. The aim of this study is to better understand the requirements for processes and material properties in relation to lubricant sensitivity [29,30,31,32]. In a batch process, conditions such as mixing time, lubricant quantity, and mixing speed can be adjusted easily to account for variations in lubricant action. In a continuous process, the sensitivities to lubricants have not been deeply investigated yet, and further research is required to understand the interaction of process settings in combination with excipients. This is the first time that a quantitative comparison has been made between the lubricant sensitivity in batch and continuous blending.
Download the full article as PDF here Lubricant Sensitivity of Direct Compression Grades of Lactose in Continuous and Batch Tableting Process
or read it here
Materials
A granulated lactose (SuperTab® 30GR, DFE Pharma, Goch, Germany) and an anhydrous lactose (SuperTab® 22AN, DFE Pharma, Goch, Germany) were blended with magnesium stearate (Ligamed MF-2-V, Peter Greven, Bad Muenstereifel, Germany) and FD&C blue #2, Indigo Carmine Aluminium Lake (Colorcon, Harleysville, PA, USA), as a model for an API. The tablets contained 1% w/w of the model API, 1–3% w/w of the lubricant, and the remaining 96–98% w/w was lactose.
Hebbink, G.A.; Janssen, P.H.M.; Kok, J.H.; Menarini, L.; Giatti, F.; Funaro, C.; Consoli, S.F.; Dickhoff, B.H.J. Lubricant Sensitivity of Direct Compression Grades of Lactose in Continuous and Batch Tableting Process. Pharmaceutics 2023, 15, 2575. https://doi.org/10.3390/pharmaceutics15112575
Read more on Lubricants – Pharmaceutical Excipients here:


