SARS-CoV-2 vaccine excipients polyethylene glycol and trometamol do not induce mast cell degranulation, in an in vitro model for non-IgE-mediated hypersensitivity
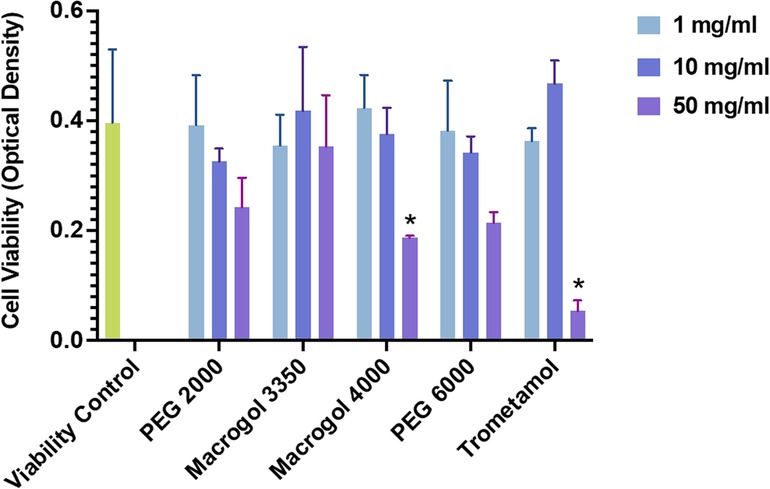
The development of vaccines against SARS-CoV2 brought about several challenges, including the management of hypersensitivity reactions to these formulations. The search for underlying mechanisms involved in these adverse events initially focused on excipients which may trigger mast cell activation responses via non-IgE pathways: polyethylene glycol and trometamol. We sought to determine whether these components, in their pure form, were capable of stimulating mast cells directly. To test this hypothesis, we used an in vitro model for non-IgE-mediated activation that has previously shown degranulation responses induced via MRGPRX2 with known drug agonists of the receptor. Human LAD2 mast cells were incubated with different concentrations (1, 10, 50 mg/ml) of trometamol and of purified polyethylene glycol/Macrogol (molecular weights: 2,000, 3,350, 4,000, and 6,000).
Mast cell degranulation was assessed using a beta-hexosaminidase read-out. Interestingly, degranulation responses for all reagents tested showed no significant differences from those obtained from the negative control (basal degranulation). Receptor-silencing assays were therefore not conducted. In summary, purified PEG and trometamol did not induce mast cell degranulation in this in vitro model for the study of non-IgE mechanisms of drug hypersensitivity, previously shown to be useful in the investigation of MRGPRX2 ligands. Studies using complete vaccine formulations, lipid conjugates, and receptor gene variants are needed to further clarify mechanisms of vaccine hypersensitivity.
Introduction
The fast-paced development of vaccines against SARS-CoV2 brought about new challenges for clinicians and allergists, including the occurrence of hypersensitivity reactions to these formulations. Regarding immediate-type reactions, symptoms ranging from mild to severe were reported, and the search for underlying pathogenic mechanisms is ongoing. Initially, focus turned towards excipients present in SARS-CoV2 vaccines that are capable of triggering hypersensitivity (1–3). Two compounds have received most of the attention: polyethylene glycol (PEG), a polymer compound found in the Pfizer-BioNTech and Moderna COVID-19 vaccines, and tris (hydroxymethyl)aminomethane (also known as trometamol, or tromethamine), an organic amine found in the Moderna vaccine. Polysorbate 80, structurally related to PEG with potential cross-reactivity (4), is found in both the Astra-Zeneca and Janssen COVID-19 vaccines.
PEG, PEGylated products, and polysorbates, which share similar structures, have been identified as inducers of hypersensitivity reactions, mostly of immediate-type (4, 5), with clinical characteristics compatible to those produced by SARS-CoV2 vaccines. The involved mechanism of hypersensitivity is only partially understood. Recent studies showing cross-reactivity between different pegylated compounds (4, 5), and the identification of IgE against PEG in the context of anaphylaxis (6), as well as evidence of antigen-specific inhibition of histamine release by monomeric and dimeric fractions of PEG (7), suggest that an immunoglobulin E (IgE)-mediated pathway is involved. On the other hand, variable results have been observed in terms of skin testing positivity to PEG and PEG-related products (4), and adverse reactions often occur upon first documented exposure to a specific PEG-containing formulation. The other compound, trometamol, has been described as a causative agent of anaphylaxis in gadolinium-based formulations (8). Positive skin-testing with trometamol has recently been described in patients with delayed local reactions to the Moderna mRNA SARS-CoV2 vaccine (9). Interestingly, these patients experienced earlier-onset, but milder, local reactions after their second vaccine dose (9). Larger studies regarding hypersensitivity mechanisms for trometamol are missing. Finally, a publication by Krantz et al., reports tolerance to a second dose of a SARS-CoV2 vaccine in a cohort of 189 patients who experienced immediate reactions to a first dose of the vaccine (10). Given the evidence described above, we believe that an exploration of non-IgE mechanisms for vaccine hypersensitivity is justified.
Recently, our group has shown that several drugs are capable of inducing mast cell (MC) degranulation in vitro directly, as measured by beta-hexosaminidase release (11). Furthermore, receptor silencing experiments showed that many of these compounds induce such activation via the Mas-related G protein-coupled receptor member X2 (MRGPRX2), a receptor found mainly in connective tissue MCs and identified as a cause for pseudo-allergic, immediate-type reactions to drugs, as well as other immune and allergic conditions (11). MRGPRX2 is also a known target for specific drug groups with common structural elements (11). The newly demonstrated relevance of this receptor in drug hypersensitivity highlights the importance of investigating non-IgE pathways for MC activation by drugs and excipients. We therefore considered it important to investigate whether PEG and trometamol are capable of stimulating MCs directly. To meet this objective, we used a model for non-IgE-mediated activation that has previously that has previously revealed degranulation responses via MRGPRX2 (11). The model employed requires two steps. First, to observe whether compounds activate MCs directly, in a way not specific to any receptor, by characterizing the degree of degranulation induced in human MCs (LAD2 cell line), by the presence of drugs in incubation. Second, if degranulation is found, to demonstrate the involvement of a specific receptor pathway by confirming its presence in the cell and then silencing it. In the case of MRGPRX2, this involves a lentiviral knock-down system and confirmation of receptor silencing using Western Blot and flow cytometry (11). The second step depends on finding activation in the first, which we completed using the method explained below.
Download the full article as PDF here SARS-CoV-2 vaccine excipients polyethylene glycol and trometamol do not induce mast cell degranulation, in an in vitro model for non-IgE-mediated hypersensitivity
or read it here
Paola Leonor Quan, Laia Ollé, Marina Sabaté-Brescó, Yanru Guo, Rosa Muñoz-Cano, Annette Wagner, Gabriel Gastaminza and Margarita Martín, SARS-CoV-2 vaccine excipients polyethylene glycol and trometamol do not induce mast cell degranulation, in an in vitro model for non-IgE-mediated hypersensitivity, BRIEF RESEARCH REPORT article, Front. Allergy, 20 December 2022, Sec. Drug, Venom & Anaphylaxis, Volume 3 – 2022, https://doi.org/10.3389/falgy.2022.1046545
See also our article What are the Ingredients and Excipients in the COVID-19 Vaccines?