Quality by design driven systematic development of nanoemulgel of clobetasol-17-propionate for effective treatment of psoriasis
Abstract
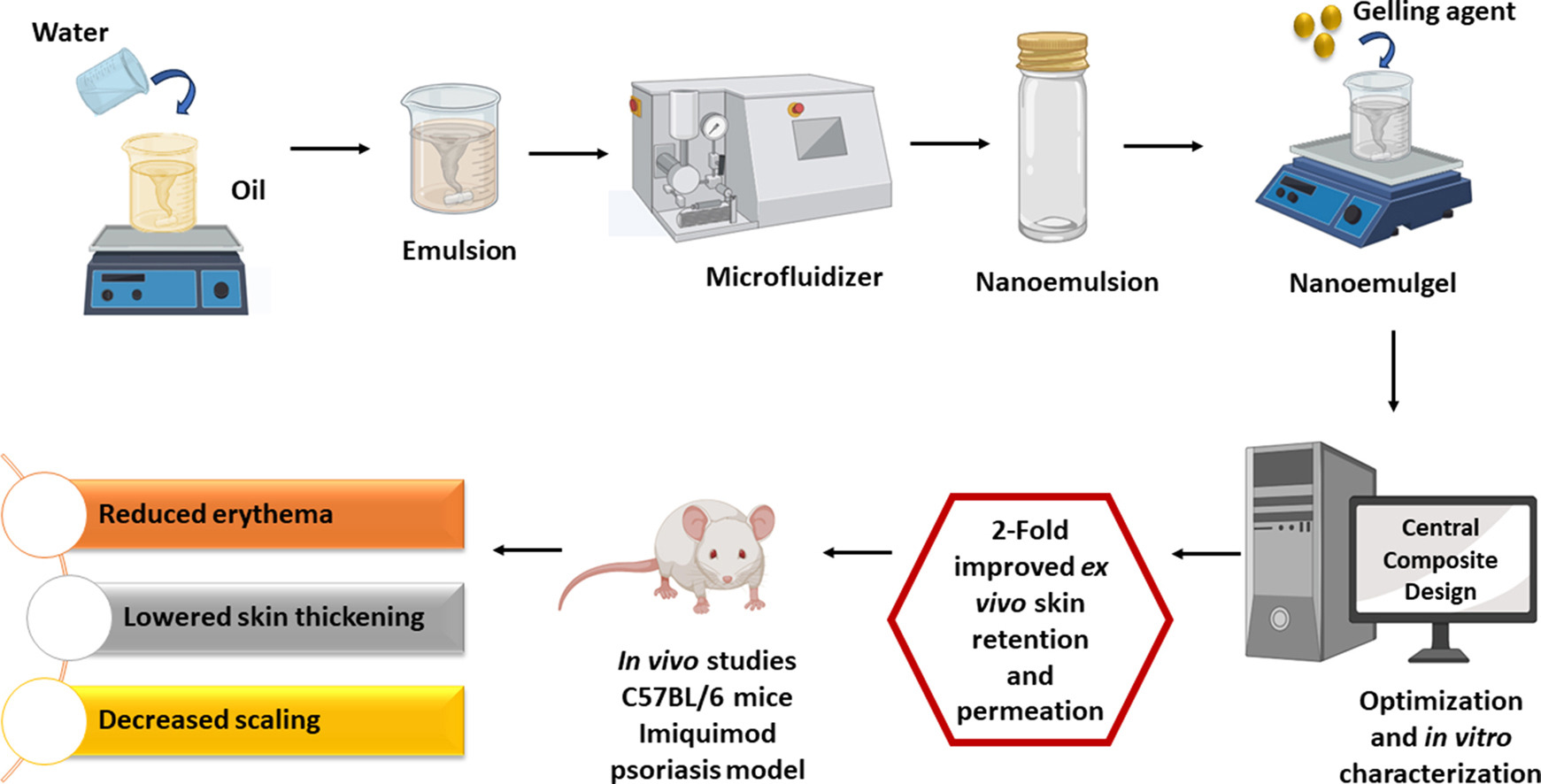
Nanoemulgels require two step formulation, the first one being formulation of nanoemulsion followed by the incorporation of nanoemulsion within a gel matrix. The current investigation aimed to formulate a Clobetasol-17-propionate (CP-17) nanoemulgel using QbD approach as anti-psoriatic for plaque type psoriasis. CP-17 was selected as a therapeutic agent to incorporate into the nanoemulsion system and then optimized CP-17 loaded nanoemulsion was incorporated into gelling agent (Carbopol 940) for topical use. Central Composite Design (CCD) was used. The independent factors used were amount of Smix (X1) and amount of gelling agent (X2). The dependent factors were in vitro drug release (Y1), droplet size (Y2) and encapsulation efficiency (Y3). The droplet size, rheological evaluation, PDI, in vitro release, ex vivo permeation and, retention and, in vivo studies were carried out. The nanoemulgel formulated was found to have droplet size within the range of 37.16 ± 21.16 nm. The ex vivo permeability was 44.68 ± 1.21 % and the skin retention was 40.86 ± 0.61 % after 7 h, both of which were greater than the marketed formulation. The CP-17 nanoemulgel showed 1.3-fold higher flux (498.66 ± 14.40 μg/cm2 h) than marketed formulation (383.9 ± 8.59 μg/cm2 h). The apparent permeability coefficient (APC) found to be 1.6 × 10−4 μg/min and 1.27 × 10−4 μg/min, respectively. The in vivo evaluation showed a reduction in PASI scores for erythema, thickness and scaling. The nanoemulgel produces sustained effect of CP-17 on the site of application as localized effect is required. The inflamed stratum corneum will demand hydration as well as targeted therapy, which the CP-17 nanoemulgel successfully provides.
Introduction
Psoriasis is a disease where the epidermis of the skin becomes red, scaly and inflamed. This affects 1–3 % of the world’s population. Psoriasis is a not life threatening but it is a painful and disabling disease. The onset of Psoriasis occurs at two distinct ages: at adolescence (16–22 years of age) and at old age (above 57 years of age) [1]. Plaque-type psoriasis is the most prevalent type of psoriasis in the world. It is an autoimmune skin disorder where there is the development of plaques or red scaly lesions. These are due to the excessive proliferation of the keratinocytes and induction of release of inflammatory mediators by TNFα and Interleukin-23 that further aggravate the symptoms [2]. Mechanism of Plaque-type psoriasis arises through the IL23/THelper 17 Axis, genetically predisposed skin when exposed to an external trigger the keratinocytes release Cathelicidin (LL-37), this complexes with the DNA and the DNA-LL-37 complex activate the plasmocytoid dendritic cells, which then stimulates the differentiation of T cells in the lymph nodes to T-Helper cells 1 and 17 [3]. The T-Helper cell 17 is associated with the release of many proinflammatory mediators that subsequently lead to formation of thick scaly and painful legions on the surface of the skin [4] (see Table 1, Table 2, Fig. 8, Fig. 9).
There is no permanent treatment to cure psoriasis but there are therapies used to manage and mitigate the scaly psoriatic lesions. Topical agents are the therapies that are first used before switching to systemic treatments. Emollients are the first line of treatment that are preferred to treat psoriatic lesions that are not wide spread i.e., less than 20 % of the body surface area. Emollients are hydrating and soften the hyperkeratotic surface of the scales; they score poorly with patients as they are greasy and sticky in nature [5]. Keratolytic agents like Salicylic acid when in combination with coal tar and topical corticosteroids increase its absorption, which ultimately soothes and reduces the hyperplasia but salicylic acid is also irritant in nature. Similarly, Coal tar given in combination with phototherapy for psoriasis is effective in reducing the scales but there is strong persistent odour associated with its use. Phototherapy by using sunburn ultraviolet rays UV- B (300–320 nm) and UV-A (320–400 nm) is also used alongside topical therapy. Coal tar used separately does cause skin cancers in rare cases [6]. The anthralin given in combination with UV-B causes staining and irritation due to free radical formation. PUVA (Psoralen and UV-A) have a risk of causing skin cancers [7]. Topical corticosteroids like Clobetasol-17-propionate (CP-17), Bethamethasone dipropionate are widely used to manage early-stage psoriasis that is less than 20 % of the body surface area. They are widely accepted and tolerated due to their efficacy and absence of skin irritation, staining and wide accessibility due to cost effectiveness.
CP-17 is classified as a super potent topical corticosteroid that is beneficial in the treatment of skin disorders such as eczema, contact dermatitis, itching, and psoriasis [8]. CP-17 shows its immunomodulatory and anti-inflammatory response through first binding with the cytoplasmic glucocorticoid receptor to form a complex, this complex then translocates into the nucleus and dimerizes into a homodimer that then binds to a corticosteroid responsive gene which inhibits the transcription of many genes that regulate the synthesis of pro inflammatory mediators like nuclear factor-k Ba (IkBa) by stimulating expression of the IkBa gene [9]. It also inhibits the transcription of genes that regulate the synthesis of IL-6,IL-1,IL-2, INF-ɣ and tumour necrosis factor (TNF-α). That play an important role in the inflammation seen in plaque type psoriasis [10]. CP-17 although being a super potent topical corticosteroid its action wears off upon repeated usage as the glucocorticoid receptor’s lose sensitivity [11]. The loss of sensitivity is due to the repeated application of conventional formulations like creams and ointments available in the market. These formulations do not have sufficient penetration and skin retention, which therefore leads to increased frequency of application by patients. This leads to repeated exposure of CP-17 to Glucocorticoid receptors, that results in tachyphylaxis or drug tolerance. To overcome this, a formulation with reduced dosing frequency (nanoemulgel) is required for optimal usage of CP-17 as the first line therapy in plaque type psoriasis. Since pain is prominently witnessed by patients a gel formulation that provides a cooling effect on the skin can increase patient compliance [12].
Nanoemulsions are biphasic liquid dispersions of two immiscible liquids where the dispersed phased contains an active ingredient solubilized into droplets and uniformly dispersed throughout the continuous phase. The globule size of the dispersed phase ranges from 10 to 500 nm [13,14]. Nanoemulsions have low viscosity and tend to wash off from the surface of application [15]. This is especially relevant in topical delivery, where penetration and retention of the therapeutic agent is required. To overcome this nanoemulsions are incorporated within a gel delivery system (hydrogel matrix of a suitable gelling agent) is called as a nanoemulgel, that can be utilized when localized action of the drug is required. Further, to formulate gels in a colloidal particle system, large amounts of aqueous or hydroalcoholic bases are used to impart thermodynamic and physical stability to the system. When nanoemulsions are incorporated into gels there is increased retention as well as modified release pattern observed [16]. The study aims at formulation of a CP-17 nanoemulgel where clobetasol can be incorporated into a system that takes the advantages of both nano formulations as well as hydrogels [17]. The hydrogels provide nanoemulsions with suitable rheological properties and the fine droplet size of nanoemulsions allow for increased skin penetration and the oil phase accounts for increased skin retention [18,19]. The study uses Quality by Design approach to formulate CP-17 nanoemulgel. Formulation of a nanoemulgel is a two-step process where the nanoemulsion formulation directly affects multiple parameters of the resulting nanoemulgel.
Read the full article here
Materials
Clobetasol-17-propionate (CP-17) was provided by Sanjivani Pharma Pvt. Ltd. The excipients like Oleic Acid, Tween-80, Triethanolamine, potassium hydroxide, sodium hydroxide and Carbopol 940 were purchased from ResearchLabs Fine Chemical Industries (Mumbai, India). Methanol was purchased from Advent Chembio Pvt. Ltd. (Navi Mumbai, India). Double distilled water was used throughout the study.
Shanaika Devadiga, Ashwini Sermasekaran, Alok D. Singh, Surendra Agrawal, Sanjay Sharma, Deepak Choudhary,
Quality by design driven systematic development of nanoemulgel of clobetasol-17-propionate for effective treatment of psoriasis, Journal of Drug Delivery Science and Technology, 2024, 105422, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105422.
Read more on Topical Excipients as Pharmaceutical Excipients here:


