Nanoformulations for dermal delivery of Imiquimod: The race of “soft” against “hard”
Abstract
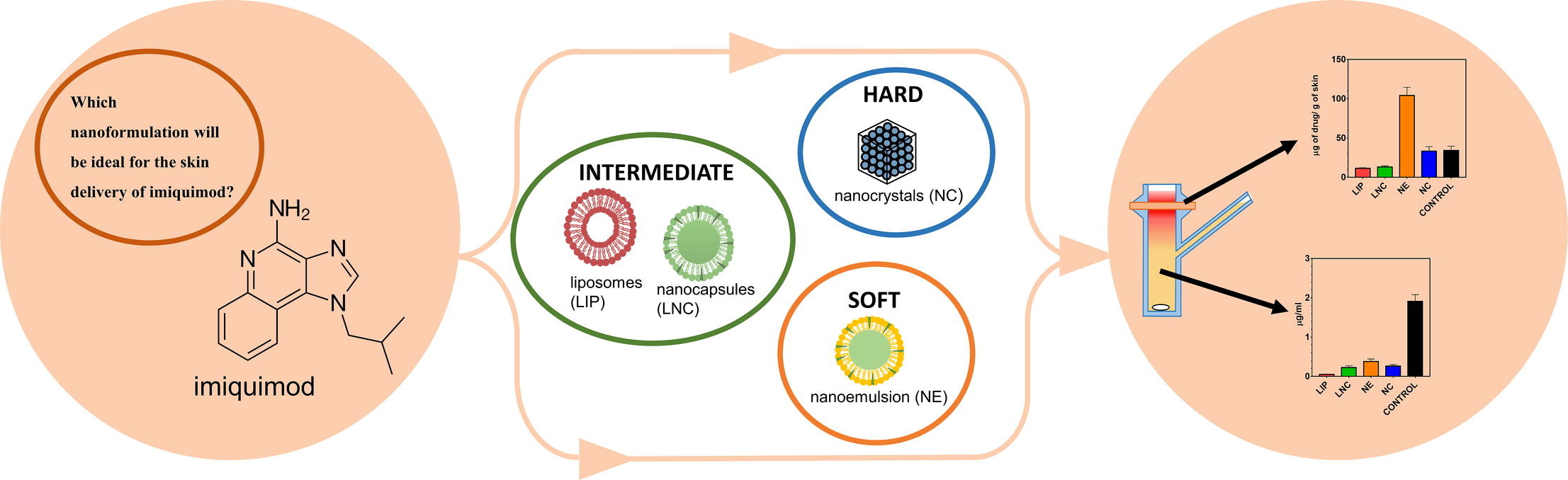
Imiquimod (IMQ) is an immunostimulating agent used in the treatment of basal cell carcinoma and actinic keratosis. Due to its low solubility and poor skin bioavailability, the dermal formulation of IMQ remains challenging. In analogy to tyre compounds used in Formula 1 racing, we compare four types of nanosystems belonging to three groups: (i) “hard” nanoparticles in the form of IMQ nanocrystals, (ii) “intermediate” nanoparticles in the form of liposomes and lipid nanocapsules, and (iii) “soft” nanoparticles in the form of a nanoemulsion based on oleic acid. The nanoemulsion and nanocrystals were able to incorporate the highest amount of IMQ (at least 2 wt%) compared to liposomes (0.03 wt%) and lipid nanocapsules (0.08 wt%). Regarding size, liposomes, and lipid nanocapsules were rather small (around 40 nm) whereas nanocrystals and nanoemulsion were larger (around 200 nm).
All developed nanoformulations showed high efficiency to deliver IMQ into the skin tissue without undesirable subsequent permeation through the skin to acceptor. Especially, the 2 wt% IMQ nanoemulsion accumulated 129 μg/g IMQ in the skin, compared to 34 μg/g of a 5 wt% commercial cream. The effects of the respective nanoparticulate systems were discussed with respect to their possible diffusion kinetics (Brownian motion vs. settling) in the aqueous phase.
Introduction
Basal cell carcinoma (BCC) is one of the most frequently diagnosed types of skin cancer, with an increasing incidence. Although it is rarely malignant and the associated mortality is relatively low, it has a large impact on life quality. Surgical biopsy is the most common form of treatment, but it is not always possible, for example when the eye area is affected, or the tumors are unevenly distributed throughout the body. In such cases, dermal application of actives is required either in the treatment of the tumours themselves or of actinic keratosis, the pre-tumor stage (Esmann and Jemec, 2014).
One of the main drugs used in the treatment of BCC and actinic keratosis is imiquimod (IMQ, see Table 1) (Waalboer-Spuij et al., 2015). IMQ activates a nonspecific immune response (e.g., NK cells and macrophages) which stimulates cytokine secretion and induces apoptosis in affected cells. However, due to the abnormal increase in cytokines in the bloodstream after oral administration of even a minimal dose, the dermal application of IMQ is the only option (Schön and Schön, 2007, Stanley, 2002). Unfortunately, the delivery of IMQ from standard formulations (creams, gels) to the deeper skin layers is often insufficient. The main reason is the extremely low solubility of IMQ in most pharmaceutical excipients, including water (18 µg/ml) (Lapteva et al., 2019). For these reasons, IMQ formulation is highly challenging.
To increase the bioavailability of poorly soluble drugs into the skin tissue, innovative nanocarrier systems can be used. Various nanoparticle formulations have been demonstrated for targeting drugs into skin tumour cells (Dianzani et al., 2014, Hafeez and Kazmi, 2017, Chung et al., 2013, Paolino et al., 2012). Nanoparticles with a large specific surface area generally allow close contact with the SC and facilitate drug absorption into the skin. Increased drug solubility in colloidal nanoparticulate systems also contributes to improved skin delivery (Jenning et al., 2000, Schäfer-Korting et al., 2007). Furthermore, nanoparticles can create local depots for drug release in the deeper skin layers; namely, hair follicles have been extensively discussed as depots for encapsulated drugs (Patzelt et al., 2017). In terms of materials, nanoparticles used in skin drug delivery usually consist of mixtures of polymers, lipids, or surfactants with the active substance, formulated in an aqueous medium. Surfactants can also serve as permeation enhancers to increase the drug permeation rate into the skin (Moghadam et al., 2013, Teixeira et al., 2014). However, their concentration must be chosen very carefully due to the risks of irritating the skin and even disrupting the skin barrier.
Several studies reported successful encapsulation of IMQ in different nanoparticulate systems. Most of them were based on oleic acid thanks to the high IMQ solubility. Particularly, microemulsions were well described and their efficacy was demonstrated in comparison with a commercially available product (Panoutsopoulou et al., 2022, Telò et al., 2016). Oleic acid was used to modify micelles to increase the concentration of IMQ in the formulation (Ghezzi et al., 2021). IMQ was also successfully encapsulated into nanodispersed emulsion gel (Stein et al., 2014), Eudragit® (Dias et al., 2018), caprolactone nanoparticles (Gazzi et al., 2020) enriched by copaiba oil or in nanosponges (Argenziano et al., 2019). Most of these studies focus on developing only one type of nanoparticles and monitoring their effectiveness, but there is still a lack of systematic comparison of different systems.
Due to the diversity of nanoformulations described in the literature and often incomparable measures of their performance, the choice of a specific drug delivery system for a specific active ingredient often resembles guesswork rather than a rational formulation design process. The aim of the present study, therefore, was to carry out a direct comparison of four different nanosystems for IMQ and evaluate their relative performance. The chosen nanosystems fall into three categories: (i) “hard” particles in the form of IMQ nanocrystals, (ii) “intermediate” nanoparticles in the form of liposomes and lipid nanocapsules, and (iii) “soft” particles in the form of a nanoemulsion. The formulations were first compared based on their physico-chemical characteristics, namely particle size, shape, polydispersity index, zeta potential (surface charge) and drug load. Then, the efficiency of these systems as IMQ drug carriers was compared in an ex vivo study on porcine skin. Two main parameters were evaluated: the total quantity of IMQ accumulated in the skin, and the quantity penetrating through the skin. The results obtained for nanoformulations were compared with those obtained using a commercial IMQ cream. Simultaneously, the likely mechanism of nanodroplet transport to the skin has been proposed.
Read more here
Materials
Imiquimod was purchased from Cayman Chemical (Michigan, USA). Phospholipon® 90 G (PL) was kindly provided by Phospholipid GmbH (Köln, Germany) and Miglyol 812 N (MCT) by IOI Oleo GmbH (Witten, Germany). Oleic acid (OA), Tween 80 (T80), Kolliphor HS 15, cholesterol, methanol (MeOH), chloroform, acetonitrile (ACN), sodium chloride, citric acid, disodium phosphate dodecahydrate, propylene glycol, gentamicin sulfate and phosphate-buffered saline (PBS) tablets were obtained from Merck KGaA
Eliška Petrová, Stanislav Chvíla, Martin Balouch, František Štěpánek, Jarmila Zbytovská, Nanoformulations for dermal delivery of Imiquimod: The race of “soft” against “hard”, International Journal of Pharmaceutics, 2023, 123577, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123577.

