High-performance nanomesh-structured cellulose as a versatile pharmaceutical excipient
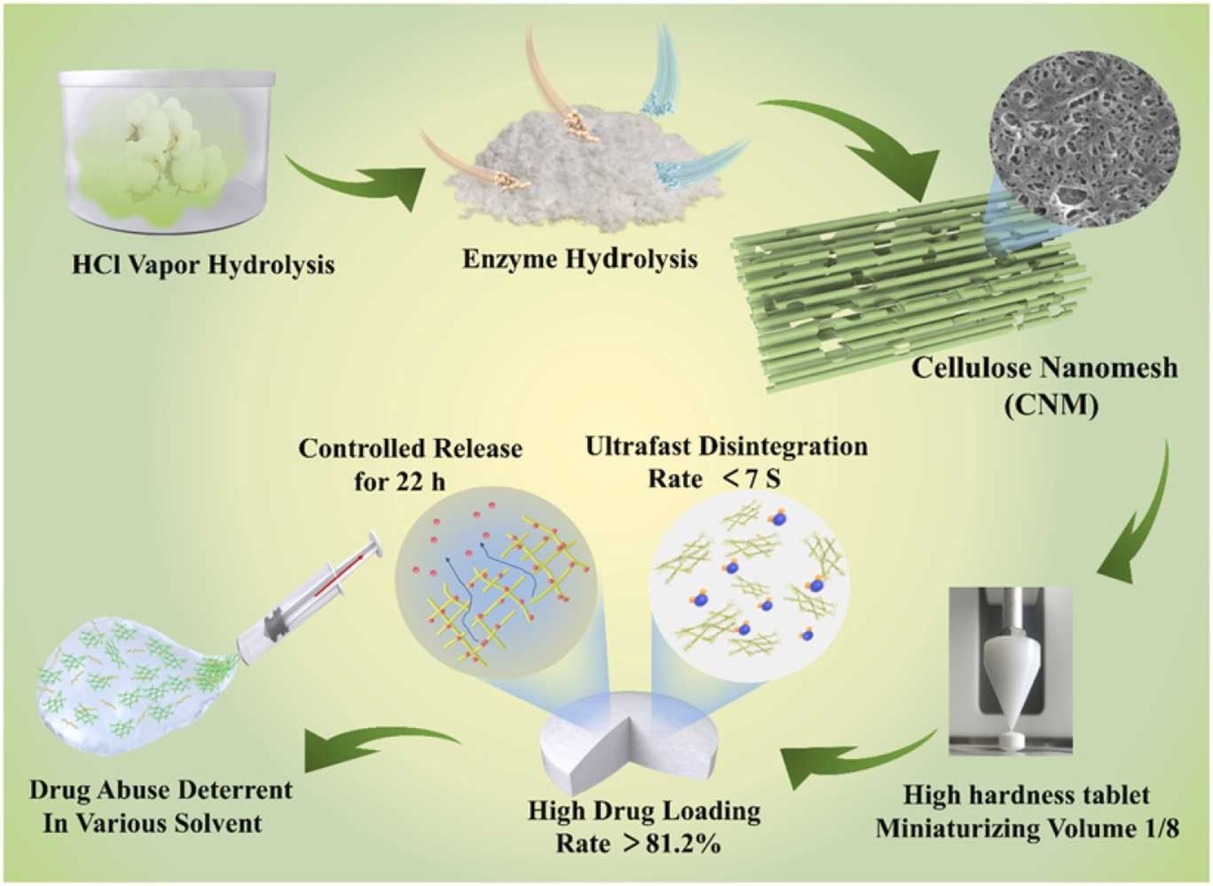
Biocompatible cellulose has been used as a pharmaceutical excipient for several decades. However, there are still shortcomings, such as low drug loading, slow disintegration, and easy drug extraction. Herein, we describe a novel process for manufacturing cellulose nanomesh (CNM), a new structured nanocellulose with a high surface area of 98.37 m2g−1 and a high yield above 88%. This process combines mild acid vapor with a dilute enzymatic hydrolysis preparation method. The unique nanomesh structure clearly demonstrates benefits as superior drug carriers, including a high drug loading rate (>81.2%), miniaturizing volume, ultrafast disintegration time (<7 s), long-term sustained release (22 h), and also a strong drug-abuse deterrent effect. Our work suggests that the CNM produces great potential as a promising multi-functional drug carrier to realize tablet miniaturization, drug abuse-deterrent, and drug slow and controlled release.
Introduction
Oral solid preparations have the advantages of easy dose regulation and inexpensive production costs, and are the most acceptable method of administration for patients (Goyal et al., 2017). Sustained-release drug technology is current research hotspot in the field of oral solid preparations (Hai et al., 2019). An ideal sustained drug release allows the release rate of highly water-soluble drug compounds to match the human circadian rhythm, thereby reducing the number of daily doses to patients, improving patient compliance, and minimizing drug side effects (Maderuelo et al., 2011, Natarajan et al., 2014). However, current sustained-release preparations of pharmaceutical excipients are usually used in combination with synthetic polymers (Dashevsky et al., 2005, Iqbal et al., 2021), which significantly increase the tablets volume and makes it difficult for patients to swallow tablets without water; prevents its use for people with dysphagia, including the elderly, infants, and patients with diseases such as Parkinson’s and cerebral palsy (Carnaby-Mann and Crary, 2005). Therefore, it is of great significance to develop and design pharmaceutical excipients with biosafety, high drug-loading rates, and sustained-release effects.
Renewable, biodegradable, and biocompatible natural polymer materials such as cellulose have been developed as pharmaceutical excipients (Si et al., 2021). Microcrystalline cellulose (MCC), prepared by dilute acid hydrolysis of cellulose, is the most representative class of natural polymer pharmaceutical excipients (Thoorens et al., 2014). However, because of the lack of structural design, MCC has a low specific surface area (<5 m2 g−1) (Ardizzone et al., 1999, Hazwan Hussin, 2018), low drug loading rate (<10%) (Song et al., 2010), and limited sustained-release effect; therefore, it is only used as a filler or diluent (Bolhuis and Armstrong, 2006). Furthermore, the needle-like structure of CNC may cause the potential nanotoxicity (Clift et al., 2011, Wang et al., 2019). Consequently, despite the fact that some researchers use CNC as a drug carrier to accomplish targeted drug delivery, it has not been used with pharmaceutical preparations (Brinkmann et al., 2016, Sehaqui et al., 2011). There are no reports of nanotoxicity for cellulose nanofiber (CNF) and bacterial cellulose (BC), whose length can exceed ten micrometers and whose diameter is on the nanometer scale (Pachuau, 2017).
However, due to its block or sheet structure after drying, it is mostly utilized for external dressings and is not suited for solid oral preparations (Hasibuan et al., 2021, Lin et al., 2013). Therefore, the development of cellulose with a high specific surface area, fast disintegration rate, and superior biosafety is expected to replace the current widely used pharmaceutical excipients. A feasible way to achieve this goal is to directly excavate pores on the cellulose substrate so that the cellulose material may have a macroscopic scale of micrometers and a large number of nanopores on the microscopic scale. This hypothesis has been tried to be realized in a number of ways, but none have proved successful. This can be as a result of the weak hydrolysis strength, which makes it challenging to drastically alter the morphology of cellulose and increases the likelihood of obtaining MCC (Zhao et al., 2018); contrarily, intense hydrolysis will significantly dissociate the cellulose morphology, resulting in the final hydrolysis product as CNC (Melikoglu et al., 2019). Therefore, choosing a suitable hydrolysis method to make the hydrolysis intensity moderate may be the key point for directly excavating the pores on the cellulose matrix.
In view of aforementioned assumption, for the first time, a novel structured cellulose nanomesh (CNM) was successfully created using a quick, inexpensive method called dilute HCl vapor hydrolysis combined with enzymatic hydrolysis (DAVE) processes. This method has mild hydrolysis conditions, but can effectively excavate pores directly on the cotton linter cellulose. Then, we investigated the CNM drug loading, sustained and controlled drug release, and drug abuse-deterrent properties.
Read more
Xiaowen Li, Dongdong Ye, Zhongrun Xiang, Huai Wang, Huiqing Wang, Yun Lu, Risheng Yao, High-performance nanomesh-structured cellulose as a versatile pharmaceutical excipient, Industrial Crops and Products, Volume 197, 2023, 116580, ISSN 0926-6690, https://doi.org/10.1016/j.indcrop.2023.116580.

