In-situ formation of nanoparticles from drug-loaded 3D polymeric matrices
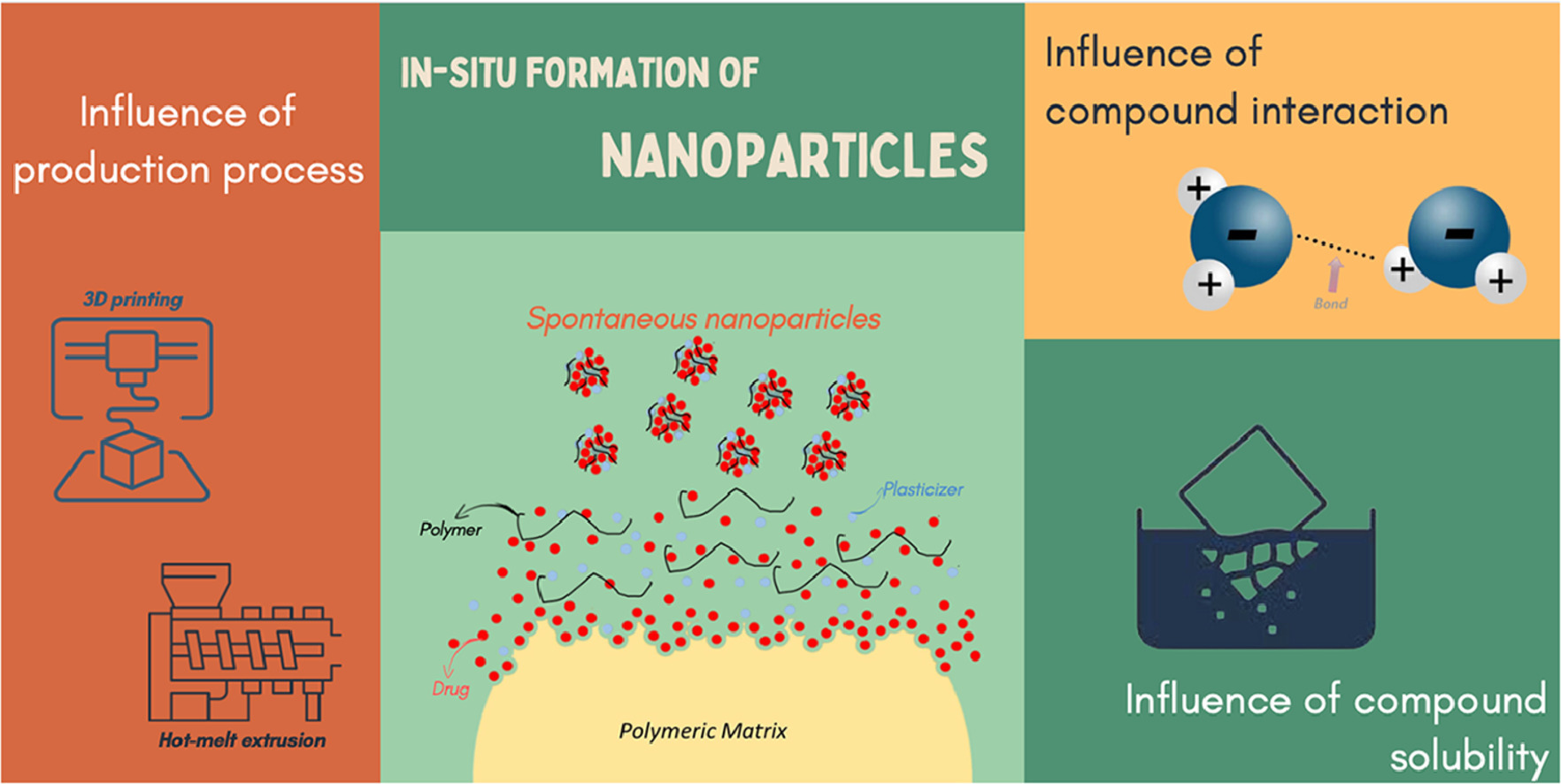
The in-situ formation of nanoparticles from polymer-based solid medicines, although previously described, has been overlooked despite its potential to interfere with oral drug bioavailability. Such polymeric pharmaceuticals are becoming increasingly common on the market and can become even more popular due to the dizzying advance of 3D printing medicines. Hence, this work aimed to study this phenomenon during the dissolution of 3D printed tablets produced with three different polymers, hydroxypropylmethylcellulose acetate succinate (HPMCAS), polyvinyl alcohol (PVA), and Eudragit RL PO® (EUD RL) combined with plasticizers and the model drug naringenin (NAR). The components’ interaction, dissolution behavior, and characteristics of the formed particles were investigated employing thermal, spectroscopic, mechanical, and chromatographic assays. All the systems generated stable spherical-shaped particles throughout 24 h, encapsulating over 25% of NAR.
Highlights
- 3D printed tablets spontaneously generate drug-loaded nanoparticles in aqueous medium
- In-situ nanoparticles formation is caused by drug-plasticizer-polymer interactions
- Stable spherical-shaped nanoparticles were produced with high drug encapsulation
- Solubility and formulation treatment are relevant for nanoparticle formation
- In-situ formation of nanoparticles should be monitored in 3D polymeric systems
Results suggest encapsulation efficiencies variations may depend on interactions between polymer-drug, drug-plasticizer, and polymer-plasticizer, which formed stable nanoparticles even in the drug absence, as observed with the HPMCAS and EUD RL formulations. Additionally, components solubility in the medium and previous formulation treatments are also a decisive factor for nanoparticle formation. In particular, the treatment provided by hot-melt extrusion and FDM 3D printing affected the dissolution efficiency enhancing the interaction between the components, reverberating on particle size and particle formation kinetics mainly for HPMCAS and EUD RL. In conclusion, the 3D printing process influences the in-situ formation of nanoparticles, which can directly affect oral drug bioavailability and needs to be monitored.
Download the full article as PDF here In-situ formation of nanoparticles from drug-loaded 3D polymeric matrices
or read it here
Materials
NAR [(2S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one, purity ≥ 98%, lot MKCD1056] was obtained from Sigma-Aldrich (St. Louis, MO, USA). The polymer PVA (Parteck® MXP, lot F1952064) was donated by Merck (Darmstadt, Germany). HPMCAS (lot SF60G410004) and Eudragit RL PO® (EUD RL, Poly(ethyl acrylate-co-methyl methacrylate-co-trimethylammonioethyl methacrylate chloride), lot G170936626) were donated by Ashland Specialty Ingredients (Covington, LA, USA), and Evonik industries (Darmstadt, Germany), respectively. The plasticizers glycerin (GLY, lot 58591) and triethyl citrate (TEC, lot S7425151) were purchased from Dinâmica® (São Paulo, Brazil) and Merck (Darmstadt, Germany), respectively. All other chemicals and solvents were of analytical grade
Felipe Q. Pires, Idejan P. Gross, Livia L. Sa-Barreto, Tais Gratieri, Guilherme M. Gelfuso, Sonia N. Bao, Marcilio Cunha-Filho, In-situ formation of nanoparticles from drug-loaded 3D polymeric matrices, European Journal of Pharmaceutical Sciences, 2023, 106517, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2023.106517.
Read more on “Pharmaceutical 3D printing” here:


