Nintedanib solid lipid nanoparticles improve oral bioavailability and ameliorate pulmonary fibrosis in vitro and in vivo models
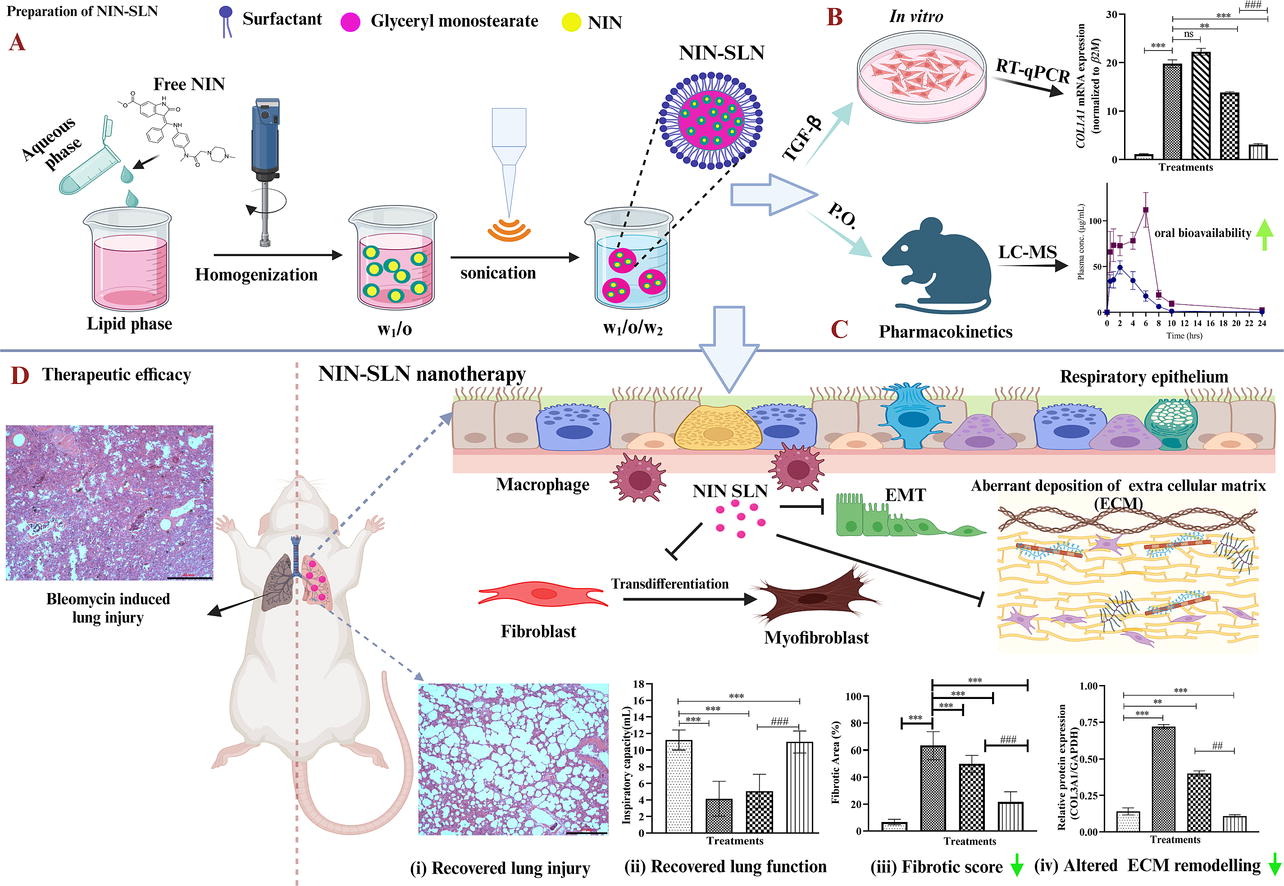
Nintedanib (NIN) and pirfenidone are the only approved drugs for the treatment of Idiopathic Pulmonary Fibrosis (IPF). However, NIN and pirfenidone have low oral bioavailability and limited therapeutic potential, requiring higher dosages to increase their efficacy, which causes significant liver and gastrointestinal toxicities. In this study, we aimed to develop nintedanib-loaded solid lipid nanoparticles (NIN-SLN) to improve the oral bioavailability and therapeutic potential against TGF-β-induced differentiation in IPF fibroblasts and bleomycin (BLM)-induced lung fibrosis in rat models.
NIN-SLN was prepared using a double-emulsification method and characterization studies (Particle size, zeta potential, entrapment efficiency and other parameters) were performed using various techniques. NIN-SLN treatment significantly (p<0.001) downregulated α-SMA and COL3A1 expression in TGF-β stimulated DHLF and LL29 cells. NIN-SLN showed a 2.87-fold increase in the bioavailability of NIN and also improved the NIN levels in lung tissues compared to NIN alone.
Pharmacodynamic investigation revealed that NIN-SLN (50 mg/Kg) treatment significantly attenuated BLM-induced lung fibrosis by inhibiting epithelial-to-mesenchymal-transition (EMT), extracellular matrix remodelling, and collagen deposition compared to free NIN. Additionally, in the BLM model of fibrosis, NIN-SLN greatly improved the BLM-caused pathological changes, attenuated the NIN-induced gastrointestinal abnormalities, and significantly improved the lung functional indices compared to free NIN. Collectively, NIN-SLN could be a promising nanoformulation for the management of pulmonary fibrosis.
Read more here
Materials
Glyceryl monostearate, palmitic acid and stearic acid were purchased from Alfa Aesar (Hyderabad, India). Dialysis tubing (molecular weight cut off 12–14 kDa), trypsin-EDTA and sulfo rhodamine-B were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lecithin soy was a product of Hi Media Laboratories (Mumbai, India). Tween 80, poloxamer 188, and poly (vinyl) alcohol were purchased from Sd Fine-Chem. Ltd (Mumbai, India). Fetal bovine serum (FBS) was purchased from Gibco, (Paisley, UK).
Rajwinder Kaur, Taslim B Shaikh, Hari Priya Sripadi, Madhusudana Kuncha, U.V.R. Vijaya Sarathi, Hitesh Kulhari, Sai Balaji Andugulapati, Ramakrishna Sistla, Nintedanib solid lipid nanoparticles improve oral bioavailability and ameliorate pulmonary fibrosis in vitro and in vivo models, International Journal of Pharmaceutics, 2023, 123644, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123644.
Read more on Orally Disintegrating Tablets (ODTs) here:


