Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases
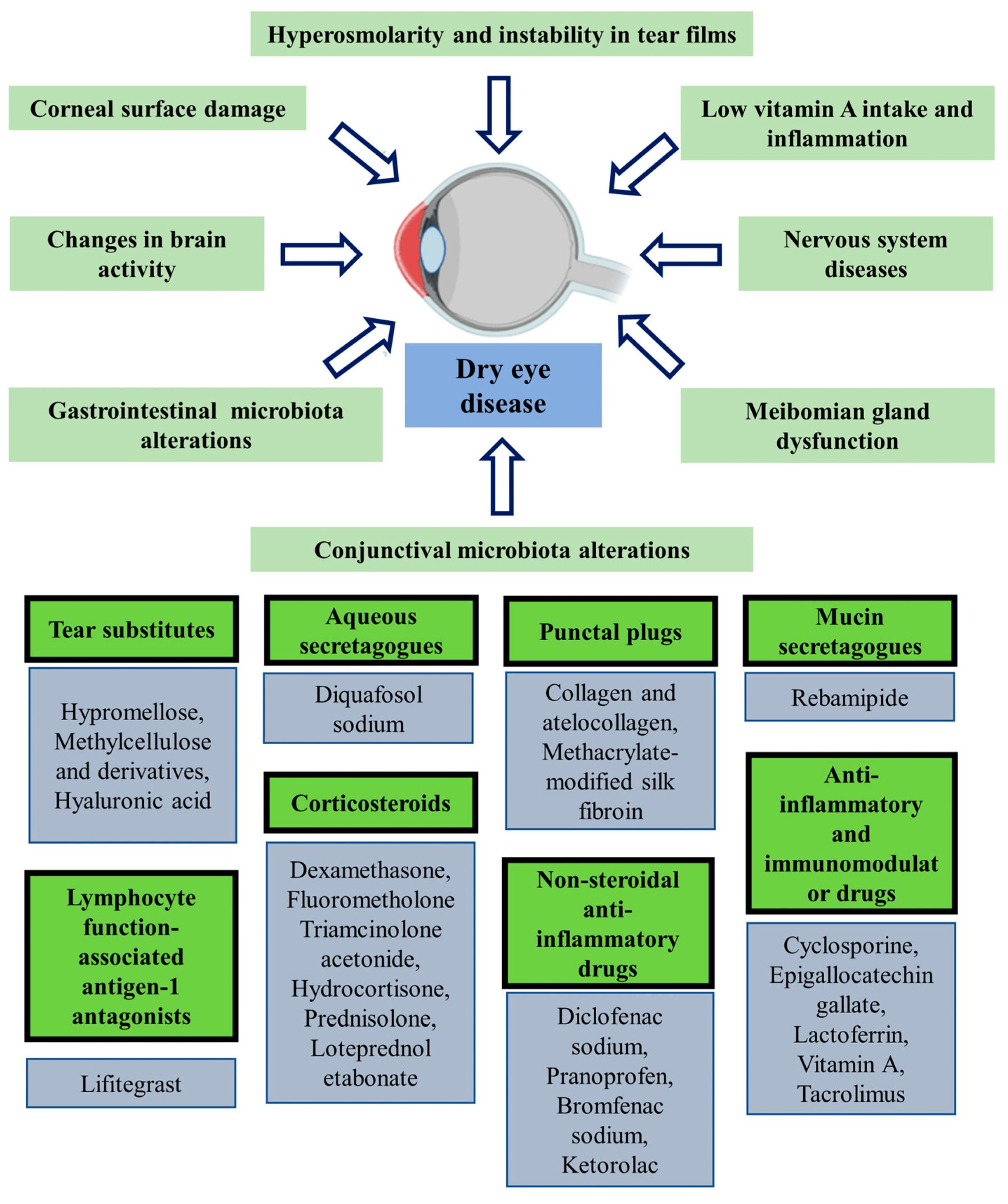
Chronic ocular diseases can seriously impact the eyes and could potentially result in blindness or serious vision loss. According to the most recent data from the WHO, there are more than 2 billion visually impaired people in the world. Therefore, it is pivotal to develop more sophisticated, long-acting drug delivery systems/devices to treat chronic eye conditions. This review covers several drug delivery nanocarriers that can control chronic eye disorders non-invasively. However, most of the developed nanocarriers are still in preclinical or clinical stages. Long-acting drug delivery systems, such as inserts and implants, constitute the majority of the clinically used methods for the treatment of chronic eye diseases due to their steady state release, persistent therapeutic activity, and ability to bypass most ocular barriers. However, implants are considered invasive drug delivery technologies, especially those that are nonbiodegradable. Furthermore, in vitro characterization approaches, although useful, are limited in mimicking or truly representing the in vivo environment. This review focuses on long-acting drug delivery systems (LADDS), particularly implantable drug delivery systems (IDDS), their formulation, methods of characterization, and clinical application for the treatment of eye diseases.
1. Introduction
Download the full review as PDF here: Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases
or read it here
Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics 2023, 15, 1746.
https://doi.org/10.3390/pharmaceutics15061746

