Polymeric microneedles for the eye: An overview of advances and ocular applications for minimally invasive drug delivery
Abstract
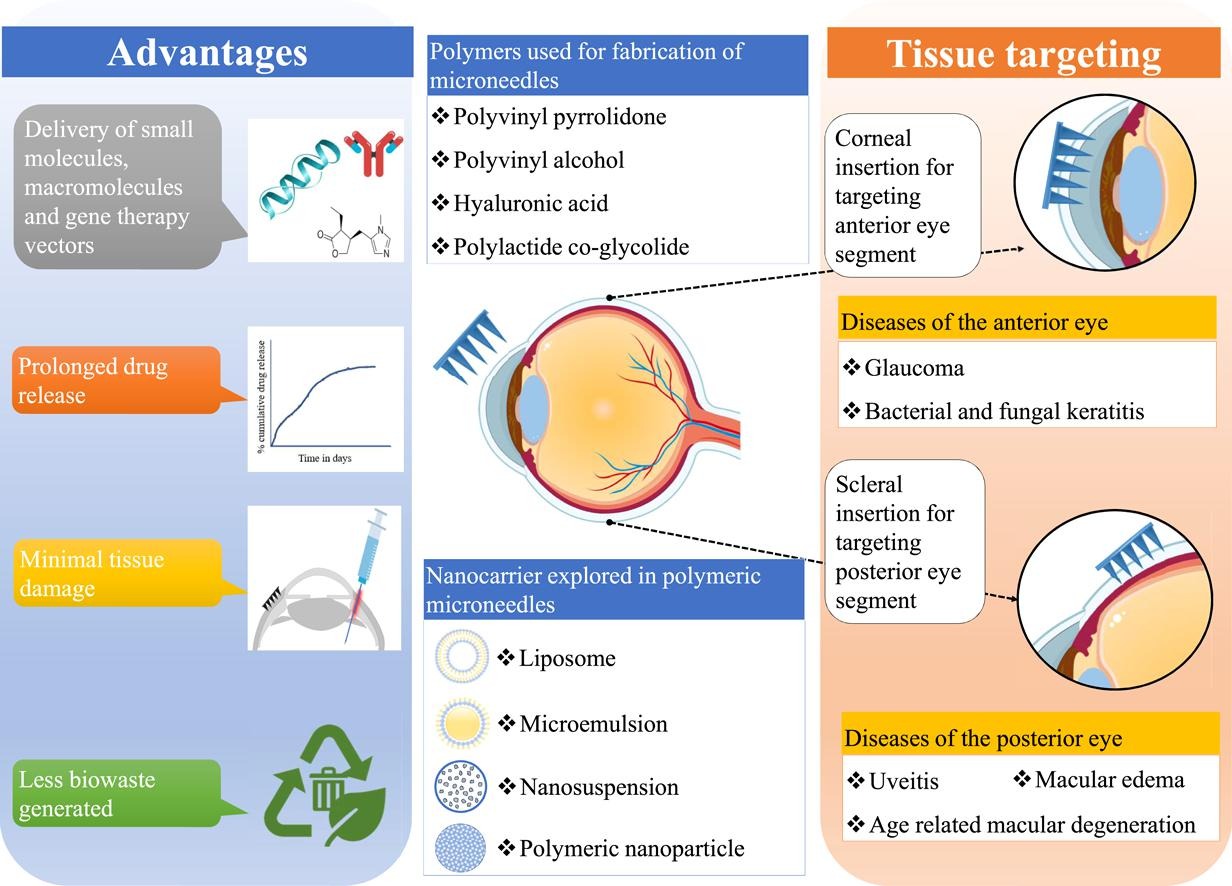
Ocular drug delivery is challenging due to the presence of tissue barriers and clearance mechanisms. Most widely used topical formulations need frequent application because of poor permeability, short retention, and low bioavailability. Invasive intraocular injections and implants that deliver drugs at the target site are associated with infections, inflammation, and even vision loss post-use. These gaps can be addressed by a delivery platform that can efficiently deliver drug with minimal tissue damage. Microneedles were introduced as a delivery platform for overcoming dermal barriers with minimal tissue damage. After the successful clinical transition of microneedles in the transdermal drug delivery, they are now being extensively studied for ocular applications. The attributes of minimally invasive application and the capability to deliver a wide range of therapeutics make microneedles an attractive candidate for ocular drug delivery. The current manuscript provides a detailed overview of the recent advancements in the field of microneedles for ocular use. This paper reviews research focusing on polymeric microneedles and their pharmaceutical and biopharmaceutical properties. A brief discussion about their clinical translation and regulatory concerns is also covered. The multitude of research articles supports the fact that microneedles are a potential, minimally invasive drug delivery platform for ophthalmic use.
Introduction
Visual impairment and low visual acuity are global health concerns. The WHO reports that over 2.2 billion people suffer from blindness globally. It is estimated that over 50 % of the cases could be prevented by proper treatments [1]. Loss of vision reduces productivity, deteriorates one’s mental health, and makes the person dependent; resulting in poor quality of life [2], [3], [4], [5], [6], [7], [8]. The leading causes of vision problems include cataracts, refractive errors, glaucoma, age-related macular degeneration, and diabetic retinopathy. Other conditions which affect the eye and visual acuity are uveitis, conjunctivitis, trachoma, onchocerciasis, dry eye syndrome, and fungal keratitis among others [9]. Age is found to be the most crucial risk factor as the risk of developing cataracts, refractive errors, glaucoma, and age-related macular degeneration was found to be greater for people above the age of 50 years. With the increase in the aging population and sedentary lifestyle, the prevalence of visual impairment is projected to increase in the next 30 years [10], [11], [12], [13], [14].
Recognizing the imperative problem of vision loss, the WHO has resolved to design and implement cost-effective and patient-centric treatment strategies for ocular health [15]. Researchers are focusing on inventing new molecules for ocular conditions as well as developing drug delivery platforms for these therapeutics [16], [17], [18]. The current clinical pipeline includes small molecules, biologics, stem cell, and gene therapy candidates along with conventional small molecules for the treatment of ocular diseases. With the advent of novel therapies, the invention of drug delivery platforms that provide desirable bioavailability and drug stability is urgently needed [16], [17], [19], [20], [21], [22], [23], [24].
Drug delivery to the eye is challenging since there exist many barriers to the penetration of the drug [25], [26]. The surface of the eye is well protected by the cornea and tear film. Anatomically 7–30 μl of tear fluid is present which is renewed at a rate of 0.5–2.2 μl/min. This results in the rapid elimination of drugs and hence tear film is the very first barrier for drug delivery to the eye. Additionally, tears are responsible to dilute the drug resulting in decreased flux. Irritation often increases tear production and blinking action causes loss of drugs from the eye. Thus, topical formulations for the eye suffer from the disadvantage of poor bioavailability and require high doses of drugs or multiple dosing to compensate for the loss [27], [28]. This increases patient discomfort and economic burden and ultimately leads to poor adherence to the therapy. Due to the presence of these barriers and limitations, it is challenging to formulate therapeutic products for effective delivery to the affected eye tissues by non-invasive topical dosage forms.
Other therapies available in the market are invasive intraocular injections and implants. The limited penetration of conventional topical preparations necessitates the use of invasive injections for the management of posterior eye disorders. Intraocular injections including intravitreal injections are required to be administered frequently to maintain the therapeutic concentration of the active moiety for a longer duration. As a result, the frequent administration of intravitreal injection elevates the risk of retinal detachment, bacterial or fungal infections, and haemorrhages [29], [30], [31]. The use of intravitreal injections subjects the patients to painful procedures and requires a clinical setting. This adds to the cost of the therapy contributing to poor patient adherence.
Researchers found that the trauma to the ocular tissue was reduced when the conventional needles used for intraocular injections were replaced with microneedles. Microneedles (MNs) are needles made using metals, non-metals, or polymers having lengths in the range of a few micrometres. They are available as single needles or as multiple projections on a single baseplate; referred to as microneedle array or microarray. which enables the delivery of drugs without causing extensive tissue damage. MNs are extensively studied as transdermal drug delivery platforms. Recently, the application of MNs in the area of ocular drug delivery are being studied [32], [33], [34], [35]. Microneedles were found to deliver drugs to the eye tissues by creating microchannels in the ocular barriers. The use of drug-loaded microneedles resulted in a significant increase in ocular bioavailability as compared to conventional eye drops [36]. This increase also translated to reduced doses and in turn marginal reduction in the overall cost of the therapy [37]. They can be self-administered, unlike conventional intraocular or intravitreal injections. Microneedles deliver the drug at precise locations in the tissues thus may reduce drug wastage. Reports suggest that microneedles decrease the risk of serious post-injection complications as compared to hypodermic needles [38], [39]. Hence, microneedles are being explored as a minimally invasive strategy for ocular drug delivery by multiple research groups globally [40], [41], [42], [43].
This review discusses the types of microneedles used for ocular applications, their formulation and design aspects, and the challenges associated with their scale-up and commercialization. In this review, we aim to collate the data from existing studies for researchers working on innovative ophthalmic formulations related to polymeric microneedles.
Read more here
Madhura Mulkutkar, Mansi Damani, Sujata Sawarkar, Polymeric microneedles for the eye: An overview of advances and ocular applications for minimally invasive drug delivery, European Journal of Pharmaceutics and Biopharmaceutics, Volume 197, 2024, 114209, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2024.114209.
Read also our introduction article on Topical Excipients here:


