Optical Coherence Tomography as a novel tool for non-invasive membrane thickness monitoring of co-extruded drug-delivery systems
Abstract
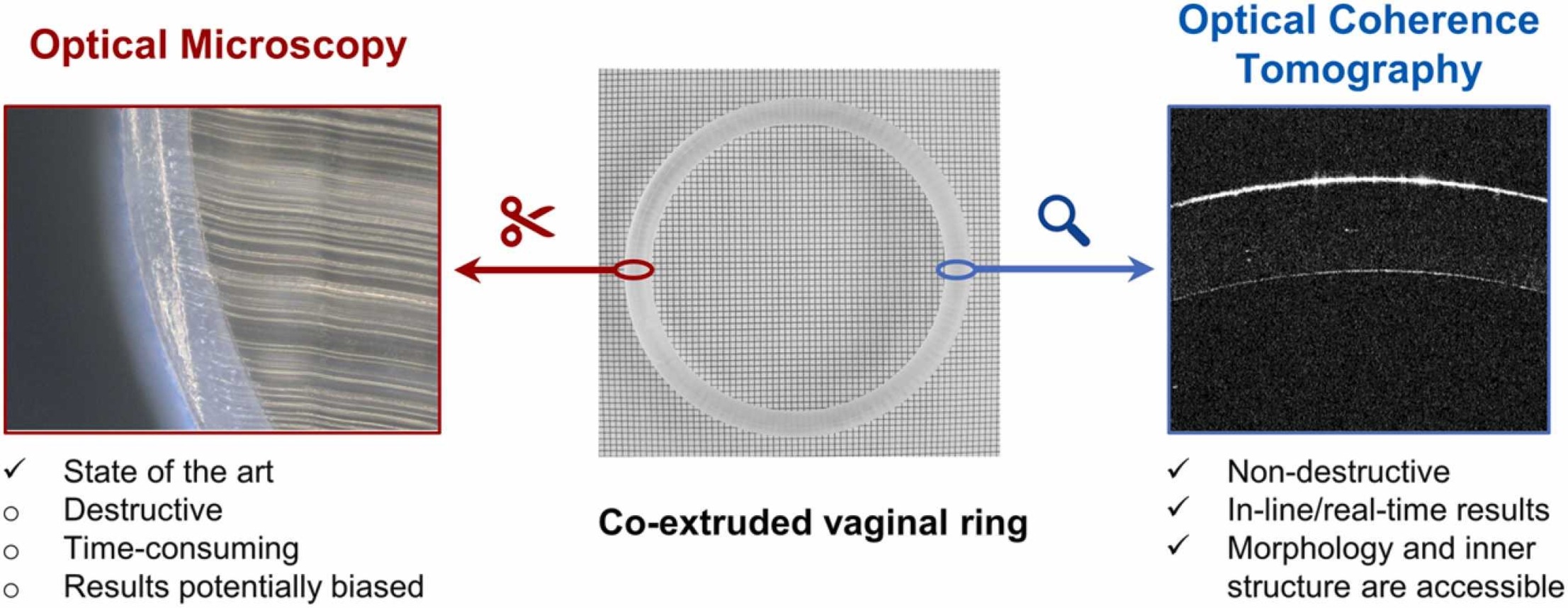
Co-extruded core-membrane formulations have been extensively explored as long-acting subcutaneous implants or vaginal inserts, guaranteeing zero-order release. Although the membrane thickness and homogeneity determine the final drug release, these parameters are still evaluated offline via destructive testing. The present study aims at tackling this shortcoming by introducing an in-line, non-invasive, fast and reproducible methodology based on optical coherence tomography (OCT) to evaluate the membrane characteristics of ethylene vinyl acetate (EVA) based co-extrudates. The novel setup enables a membrane representation and thickness analysis in real time during the manufacturing process, while the characterized membrane thicknesses are in good agreement with those obtained by optical microscopy. In addition, OCT offers novel insights into essential membrane characteristics that have not been visualized before. A case in point is the visualization of potential membrane defects, e.g. delaminated membranes, rough surfaces or viscosity mismatches in the core-membrane interface. Due to the obtained wealth of information combined with the instantaneous and destruction-free measurement, OCT has the potential to become the process analytical technology of choice for co-extruded dosage forms.
Introduction
Hot melt extrusion (HME) has been extensively used to manufacture various drug delivery systems such as (micro-) pellets, tablets, inserts and implants (Koutsamanis et al., 2020b, Narala et al., 2020, Simões et al., 2021, Spoerk et al., 2022, Zheng and Pokorski, 2021), establishing as an emerging pharmaceutical manufacturing process. Extruded dosage forms are able to extend the application space of classical solid dosage forms, as they allow embedding of an active pharmaceutical ingredient (API) into a polymer matrix, generating solid-dispersed drug carrier systems. Similar to classical dosage forms, extruded dosage forms can additionally be coated with an outer membrane to moderate and control the release behavior, whereas the coating of extruded dosage forms is mainly done in one step during the actual extrusion by a process called “co-extrusion”.
The co-extrusion process itself employs the simultaneous use of two extruders and one common die plate to manufacture co-axial drug delivery systems (Vynckier et al., 2014b). Hence, pharmaceutical co-extrusion enables the manufacturing of multilayered systems for the concurrent administration of drugs implemented in each layer (Dierickx et al., 2012, Vynckier et al., 2014a) and core/membrane or ‘reservoir-type’ formulations (Siepmann et al., 2012, Van Laarhoven et al., 2002a, Van Laarhoven et al., 2002b, Yang and Pierstorff, 2012). The reservoir systems consist of a core with the API(s) embedded in the polymer matrix and a drug-free membrane, which controls the permeation and release of the drug in the surrounding environment (Hillery and Hoffman, 2017, Siepmann and Siepmann, 2012). This design results in a constant, zero order release over long times, which, in turn, translates into high patient compliance (Browne et al., 2019, Griffin et al., 2019, Quarterman et al., 2021). Co-extrusion based reservoir systems already led to successful marketed insertable and implantable polymer-based products such as Nuvaring® and Nexplanon®, both intended for the controlled-release of contraceptive hormones over prolonged times (Friend, 2016). The drug release of such systems is mainly a function of the amount and physicochemical properties of the API as well as the core and membrane polymer characteristics (García-Estrada et al., 2021, Koutsamanis et al., 2020a, Siepmann et al., 2012, Simpson et al., 2020). For a given system, i.e., API loading and polymers used, the extent and rate of drug release falls exclusively on the membrane thickness, as this is reversely proportional to the rate of the drug released (Siepmann and Siepmann, 2012, Yang and Pierstorff, 2012). In addition to the thickness, the membrane homogeneity over the cross-section and extrudate length as well as lack of defects of the membrane are prerequisites of a high-quality and safe final product.
Commonly, the membrane thickness of co-extrudates is evaluated offline via optical microscopy or µ-CT imaging of single cross-sectional cuts (Eder et al., 2017, Tzeng and Chen, 2014, Vynckier et al., 2015). However, both methods are associated with several drawbacks: in both cases, the assessment is time-consuming and destroys the samples due to the required cuts or the exposure to ionizing radiation. Moreover, the results might not be representative for the extrusion process used, since only small fractions of a strand are investigated and manual cutting potentially induces changes in the membrane geometry due to mechanical stress. Consequently, a quick, non-destructive and reproducible method to evaluate the membrane thickness of co-extruded reservoir systems would be of high interest.
Our study closes this gap by presenting an inline, non-invasive methodology based on optical coherence tomography (OCT) to evaluate the membrane characteristics of ethylene-vinyl acetate (EVA) based co-extrudates, aiming at quicker but simultaneously more representative results. OCT is a fast and non-destructive technology based on low coherence interferometry, providing sub-surface information by reconstruction of optical echoes from structures inside semi-transparent materials. For the past 30 years, OCT has become an established technology in the field of medicine, especially in ophthalmic medical imaging (Leitgeb et al., 2021). Apart from medical applications, OCT was used to investigate pharmaceutical coating applications (Markl et al., 2014, Wolfgang et al., 2019a), shedding light on different aspects of the associated processes (Korasa and Vrečer, 2018) and their correlation to critical quality attributes of the resulting dosage forms (Wolfgang et al., 2022). Especially in pharmaceutical tablet coating processes, OCT enables the determination of the correct end-point and provides real-time information on coating thickness, coating variability, layer homogeneity and surface roughness, potentially enabling active process control (Sacher et al., 2019). However, most of the studies published so far dealt with the investigation of tablet coatings in a thickness range of up to 200 µm for clear, unpigmented functional coatings (Lin et al., 2017). Consequently, it was assumed by the scientific community that the penetration depth of OCT might not be sufficient for the investigation of co-extruded dosage forms, considering membrane thicknesses of several hundred microns and materials with considerable isotropy such as the involved polymers. The present manuscript proves the opposite by revealing that OCT is capable of reconstructing buried structures 400 µm deep in the relevant polymers by employing averaging and a suitable optical setup. Consequently, the present manuscript highlights the gained possibilities of OCT for in-line membrane analyses in pharmaceutical co-extrusion manufacturing lines.
Read more here
Matthias Wolfgang, Ioannis Koutsamanis, Martin Spoerk, Optical Coherence Tomography as a novel tool for non-invasive membrane thickness monitoring of co-extruded drug-delivery systems, Chemical Engineering Research and Design, 2023, ISSN 0263-8762, https://doi.org/10.1016/j.cherd.2023.04.052.
Read more on Lubricants – Pharmaceutical Excipients here:


