Co-release of paclitaxel and encequidar from amorphous solid dispersions increase oral paclitaxel bioavailability in rats
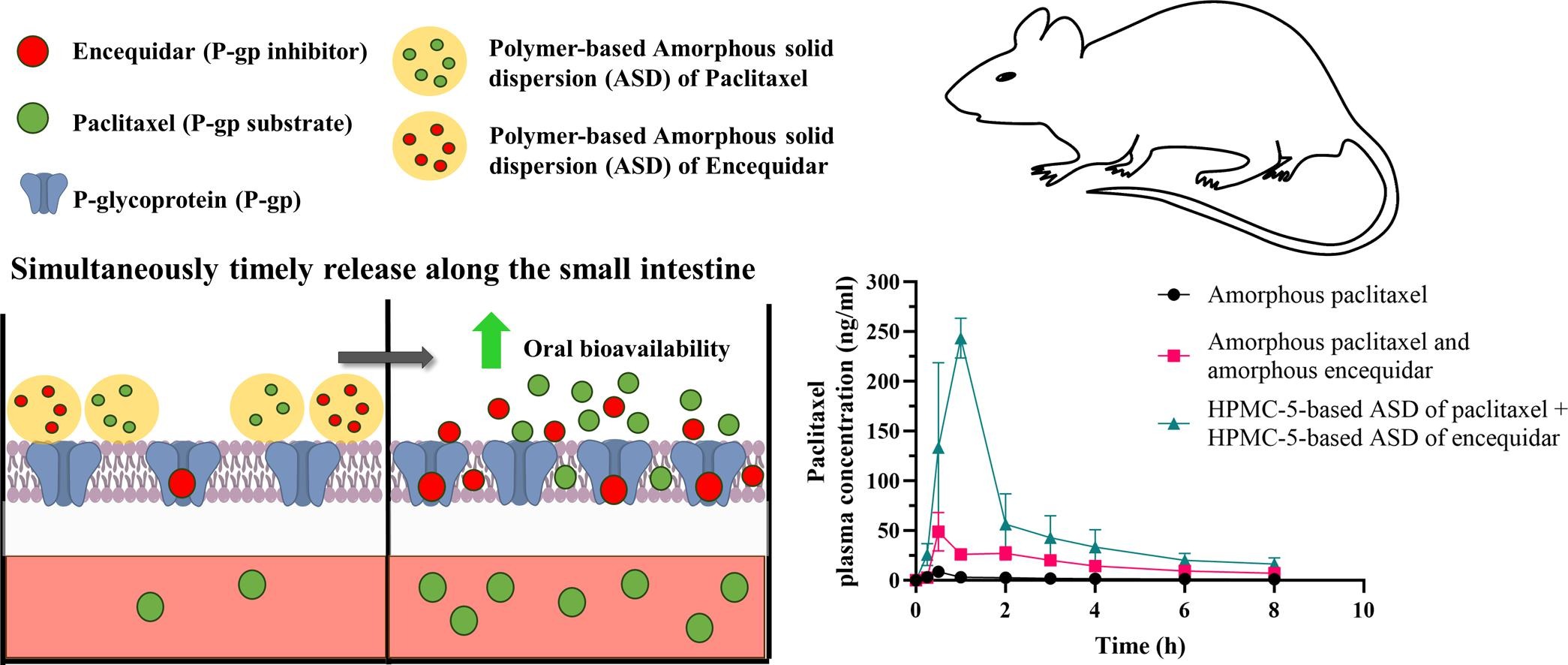
The oral bioavailability of paclitaxel is limited due to low solubility and high affinity for the P-glycoprotein (P-gp) efflux transporter. Here we hypothesized that maximizing the intestinal paclitaxel levels through apparent solubility enhancement and controlling the simultaneous release of both paclitaxel and the P-gp inhibitor encequidar from amorphous solid dispersions (ASDs) would increase the oral bioavailability of paclitaxel. ASDs of paclitaxel and encequidar in polyvinylpyrrolidone K30 (PVP-K30), hydroxypropylmethylcellulose 5 (HPMC-5), and hydroxypropylmethylcellulose 4 K (HPMC-4K) were hence prepared by freeze-drying.
Highlights
- Paclitaxel dissolution rates: ASD in PVP-K30 > ASD in HPMC-5 > ASD in HPMC-4K.
- Dissolution from amorphous solid dispersions of paclitaxel enhanced the apparent solubility.
- The presence of HPMC-5 in the intestinal lumen is more important than the dissolution rate from polymer-based ASDs of paclitaxel and encequidar when increasing the bioavailability of paclitaxel.
In vitro dissolution studies showed that both compounds were released fastest from PVP-K30, then from HPMC-5, and slowest from HPMC-4K ASDs. The dissolution of paclitaxel from all polymers resulted in stable concentration levels above the apparent solubility. The pharmacokinetics of paclitaxel after oral administration to male Sprague-Dawley rats was investigated with or without 1 mg/kg encequidar, as amorphous solids or polymer-based ASDs. The bioavailability of paclitaxel increased 3- to 4-fold when administered as polymer-based ASDs relative to solid amorphous paclitaxel. However, when amorphous paclitaxel was co-administered with encequidar, either as an amorphous powder or as a polymer-based ASD, the bioavailability increased 2- to 4-fold, respectively.
Interestingly, a noticeable increase in paclitaxel bioavailability of 24-fold was observed when paclitaxel and encequidar were co-administered as HPMC-5-based ASDs. We, therefore, suggest that controlling the dissolution rate of paclitaxel and encequidar in order to obtain simultaneous and timed release from polymer-based ASDs is a strategy to increase oral paclitaxel bioavailability.
Download the full article as PDF here: Co-release of paclitaxel and encequidar from amorphous solid dispersions increase oral paclitaxel bioavailability in rats
or read it here
Materials
Paclitaxel, free base, encequidar free base (ENC) for the in vitro study, and encequidar mesylate salt (ENC MS) for the in vivo study, were acquired from MedKoo Biosciences (Morrisville, NC, USA). Ultra-pure water was obtained from an in-house Milli Q water purification system (Merck Millipore, Burlington, MA, USA). Fasted State Simulated Intestinal Fluid V2 (FaSSIF-V2) powder was acquired from Biorelevant.com (London, UK) and FaSSIF V2 was prepared as prescribed by the manufacturer. Hydroxypropyl methylcellulose 5 (HPMC-5) was acquired from Norsk Medisinaldepot (NMD, Oslo, Norway), polyvinylpyrrolidone K30 (PVP-K30) was acquired from BASF Pharma (Ludwigshafen, Germany), and hydroxypropyl methylcellulose 4 K (HPMC-4K) was acquired from Fagron (Rotterdam, Netherlands). Tert-butanol was acquired from Sigma Aldrich (Darmstadt, Germany). Acetonitrile (HPLC graded), absolute ethanol, sodium chloride, acetic acid, and Tert-butanol were acquired from VWR Chemicals (Briare, France). Sodium hydroxide pellets and maleic acid were acquired from Merck (Darmstadt, Germany). Trifluoroacetic acid was acquired from Fisher Scientific (Loughborough, UK) and Alfa Aesar, Thermo Fischer (Kandel, Germany).
Emilie Fynbo Petersen, Bjarke Strøm Larsen, Rasmus Blaaholm Nielsen, Ils Pijpers, Dries Versweyveld, René Holm, Ingunn Tho, Jan Snoeys, Carsten Uhd Nielsen, Co-release of paclitaxel and encequidar from amorphous solid dispersions increase oral paclitaxel bioavailability in rats, International Journal of Pharmaceutics, Volume 654, 2024, 123965, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123965.
Read also our introduction article on Orally Disintegrating Tablets (ODTs) here:


