Orodispersible Films: Current Innovations and Emerging Trends
Abstract
Orodispersible films (ODFs) are thin, mechanically strong, and flexible polymeric films that are designed to dissolve or disintegrate rapidly in the oral cavity for local and/or systemic drug delivery. This review examines various aspects of ODFs and their potential as a drug delivery system. Recent advancements, including the detailed exploration of formulation components, such as polymers and plasticizers, are briefed. The review highlights the versatility of preparation methods, particularly the solvent-casting production process, and novel 3D printing techniques that bring inherent flexibility. Three-dimensional printing technology not only diversifies active compounds but also enables a multilayer approach, effectively segregating incompatible drugs. The integration of nanoparticles into ODF formulations marks a significant breakthrough, thus enhancing the efficiency of oral drug delivery and broadening the scope of the drugs amenable to this route. This review also sheds light on the diverse in vitro evaluation methods utilized to characterize ODFs, ongoing clinical trials, approved marketed products, and recent patents, providing a comprehensive outlook of the evolving landscape of orodispersible drug delivery. Current patient-centric approaches involve developing ODFs with patient-friendly attributes, such as improved taste masking, ease of administration, and enhanced patient compliance, along with the personalization of ODF formulations to meet individual patient needs. Investigating novel functional excipients with the potential to enhance the permeation of high-molecular-weight polar drugs, fragile proteins, and oligonucleotides is crucial for rapid progress in the advancing domain of orodispersible drug delivery.
Introduction
Oral medications are the preferred and widely accepted method of drug delivery due to their ease of administration, convenience for repeated and prolonged use, non-invasiveness, adaptability, scalability, and high patient compliance [1]. Nevertheless, certain patient groups, such as the elderly, children, individuals with Parkinson’s disease, and those recovering from anesthesia, often encounter challenges in swallowing or chewing solid dosage forms, particularly tablets and capsules [2]. In the United States, it is estimated that about 15 million people have dysphagia, and this represents about 4.6% of the population. Thus, extensive efforts have been undertaken to create innovative oral drug formulations that dissolve or disperse in the oral cavity, aiming to address the issue of swallowing difficulties [3]. Moreover, highly vascularized oral mucosa may increase permeability to many medications, thereby providing a rapid onset of action and increasing bioavailability, as reported elsewhere [4].
Oral disintegrating tablets (ODTs) are a type of solid oral dosage form designed to disintegrate rapidly within a matter of seconds when placed on the tongue without the necessity of water or chewing [5]. Oral thin films are a drug delivery system consisting of thin, flexible sheets that typically dissolve or disintegrate quickly, often within seconds, when placed in the mouth. They are intended to be placed either on the tongue or cheek and can be used to deliver a variety of medications, including over-the-counter (OTC) and prescription drugs [6,7].
While various names like thin strip, oral film, orally dissolving film, quick dissolve film, melt-away film, and wafer are employed to refer to the oral film dosage form, the European Medicines Agency officially designates it as an orodispersible film (ODF), or, as the United States Food and Drug Administration (U.S. FDA) commonly terms them, soluble films [8]. As per European Pharmacopeia (Ph. Eur.), ODFs are defined as sheets, either single or multilayered, composed of appropriate materials and are intended for rapid dispersion in the mouth. They rapidly disintegrate/dissolve in saliva to form a solution or suspension, thus enabling rapid absorption and delivery of the drug into the bloodstream or a rapid local effect. Moreover, ODFs offer rapid and consistent drug release, which can improve the bioavailability of some medications. The oral cavity is richly vascularized and has low enzymatic activity, which can potentially boost the bioavailability of drugs with low aqueous solubility. This route is advantageous for those drugs classified under the biopharmaceutical classification system (BCS) as Class II and Class IV. Rapid permeation across the mucosal lining of the oral cavity can circumvent acid hydrolysis in the stomach and initial hepatic metabolism. This pathway is particularly well-suited for potent medications, especially those designed for acute conditions, where they have an immediate therapeutic effect, mainly due to oromucosal and pregastric absorption, as well as direct access to the jugular vein [4]. Nevertheless, certain compounds are absorbed exclusively in the gastrointestinal tract after ingestion.
This review aims to comprehensively explore the current innovations and emerging trends of ODFs. It focuses on their design, formulation components, and manufacturing methods for potential applications in oral drug delivery. The objective is to provide a thorough understanding of ODF versatility, the recent advancements in polymers and plasticizers, and the integration of novel techniques like 3D printing. Additionally, the review seeks to emphasize the impact of incorporating nanoparticles in ODF formulations for enhanced oral drug delivery efficiency. This review adopts a systematic approach, conducting comprehensive literature searches on databases such as PubMed, Scopus, Web of Science, clinical trial databases, and patent databases. It focuses on keywords like ‘orodispersible film’, ‘polymers’, ‘manufacturing methods’, ‘3D printing’, ‘clinical trials’, ‘patents’, and ‘evaluation’. The inclusion and exclusion criteria ensure the selection of reliable studies and patents. Structured data extraction, critical appraisal, and synthesis of information are employed to present a cohesive narrative on the present advancements and evolving trends in orodispersible films while considering database-specific functionalities and intricacies.
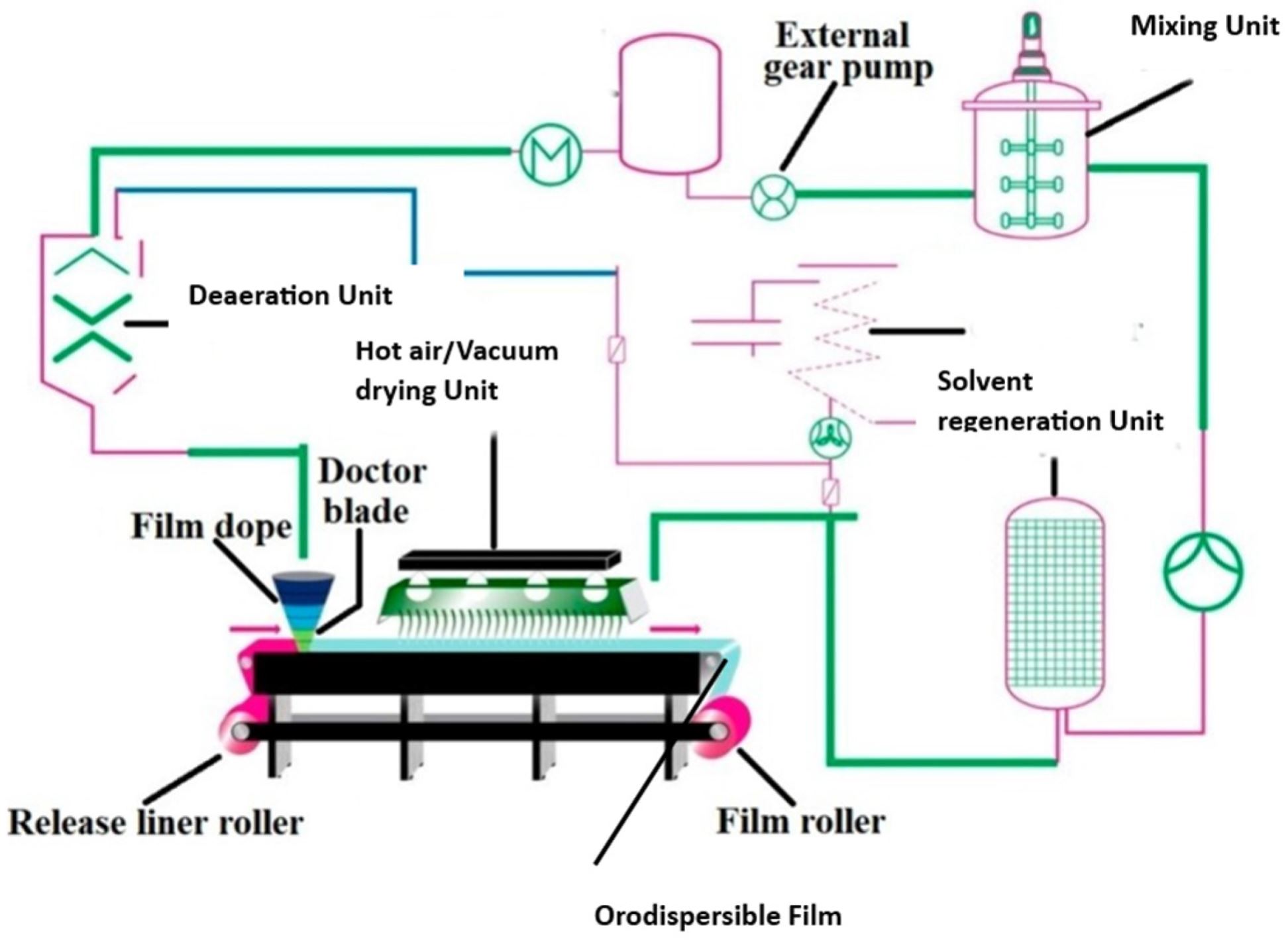
The ODF, or strip, typically employs a hydrophilic polymer, preferably with mucoadhesive properties, and aims to achieve rapid disintegration within a short period. The primary driving factors responsible for the rapid drug release of ODFs, as anticipated, are the hydration and subsequent swelling of polymers due to water diffusion [9]. The primary objective of the majority of ODFs is to quickly dissolve or disintegrate in the oral cavity, forming a solution or suspension, which is then swallowed for absorption in the gastrointestinal tract [10].
The primary constraints frequently encountered with oral films pertain to their vulnerability in high-humidity environments and their limited capacity to accommodate a substantial drug dosage. Table 1 illustrates a comparison between the advantages and drawbacks of orodispersible film drug delivery systems. The limited formulation size restricts the inclusion of additives for taste masking the drug, potentially impacting patient compliance.
Download the full article as PDF here Orodispersible Films: Current Innovations and Emerging Trends
or read it here
Polymers
Oral films primarily comprise a polymer matrix, which provides film-forming properties with structural integrity. A range of polymers, including but not limited to starch, modified starches, hydroxypropyl methylcellulose (HPMC) (including hypromellose variants E3, E5, and E15), sodium carboxymethyl cellulose (NaCMC), gelatin, hydroxypropyl cellulose (HPC), hydroxyethyl cellulose (HEC), pectin, carboxymethyl cellulose (CMC), pullulan, locust bean gum, xanthan gum, guar gum, carrageenan, povidone polymers (polyvinylpyrrolidone, PVP), polyvinyl alcohol (PVA), polyethylene oxide (PEO), maltodextrins (MDXs), and various others have been studied as potential base materials for producing ODFs [8].
The film-forming polymer, which serves as the major component of the ODF, constitutes up to 65% of the weight based on the total dry weight of the film [14]. In some cases, a combination of polymers is employed to enhance the hydrophilicity, flexibility, mouthfeel attributes, and solubility of ODFs. These polymers should be non-toxic, non-irritating, free from leachable impurities, possess excellent wetting and spreadability, have a good shelf life, and should not promote secondary infections in the oral mucosa or dental regions. Additionally, the ODFs should have adequate peel, shear, and tensile strength. Among water-soluble polymers, gelatin, and hypromellose are the most commonly used to prepare oral strips. Table 3 shows a comprehensive overview of the frequently used film-forming polymers, plasticizers, APIs, preparation methods, and the key highlights related to ODFs. The acceptability of ODFs depends upon film composition and the formation process, which affects disintegration, taste, texture, and mouthfeel attributes. The films produced should be transparent and free of air bubbles for aesthetic appeal and stability considerations.
Jacob, S.; Boddu, S.H.S.; Bhandare, R.; Ahmad, S.S.; Nair, A.B. Orodispersible Films: Current Innovations and Emerging Trends. Pharmaceutics 2023, 15, 2753. https://doi.org/10.3390/pharmaceutics15122753
Read more on “3D Printing” here:


