Influence of preparation method and choice of phospholipid on co-amorphization, physical stability, and dissolution behavior of equimolar indomethacin-phospholipid systems – A case study
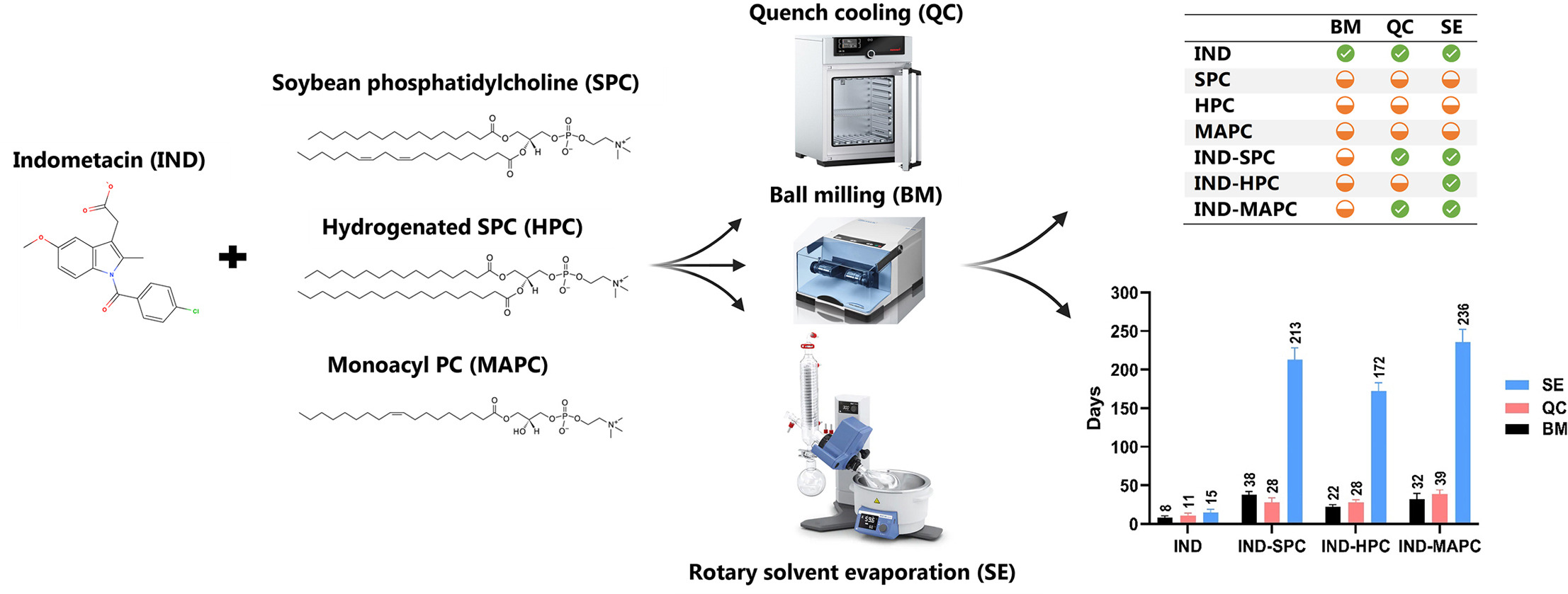
This case study aimed to evaluate the feasibility of forming equimolar co-amorphous drug-phospholipid systems by different preparation methods. Indomethacin (IND) was chosen as a model drug and combined with three phospholipids of natural origin (soybean phosphatidylcholine (SPC), hydrogenated phosphatidylcholine (HPC), and mono-acyl phosphatidylcholine (MAPC), at equimolar ratios. The systems were prepared by mechano-chemical activation-based, melt-based, and solvent-based preparation methods, i.e., ball milling (BM), quench cooling (QC), and solvent evaporation (SE), respectively. X-ray powder diffraction (XRPD), polarized light microscopy (PLM), and differential scanning calorimetry (DSC) were used to determine the solid state form of the prepared IND-phospholipid systems.
The long-term physical stability of the co-amorphous systems and the amorphous drug alone were investigated under dry conditions at room temperature. The dissolution behavior of the formed co-amorphous drug-phospholipid systems was also studied. Our findings indicate that SE-prepared co-amorphous drug-phospholipid systems can be successfully prepared across all systems, whereas the QC method could only form co-amorphous drug-phospholipid systems for IND-SPC and IND-MAPC. In contrast, the BM method failed to produce co-amorphous drug-phospholipid systems, but the drug alone could be fully amorphized.
The physical stability of SE-prepared co-amorphous drug-phospholipid systems was significantly prolonged under dry conditions (172–236 days) compared to pure amorphous IND (8–15 days) and all other systems (22–38 days). Furthermore, the co-amorphous drug-phospholipid systems formed by the SE method demonstrated improved dissolution behavior in comparison to crystalline IND, amorphous IND, and physical mixtures of IND and phospholipids. However, the phospholipids did not inhibit precipitation in the formed supersaturated IND solutions.
Download the full article as PDF here: Influence of preparation method and choice of phospholipid on co-amorphization, physical stability, and dissolution behavior of equimolar indomethacin-phospholipid systems – A case study
or read it here
Materials
SPC (Mw = 782.0 g/mol) (Lipoid® S 100, purity 98.9%), HPC (Mw = 790.0 g/mol) (PHOSPHOLIPON® 90H, purity 95.8%), and MAPC (Mw = 508.0 g/mol) (Lipoid® S LPC 80, purity 80%) were kindly donated by Lipoid GmbH (Ludwigshafen, Germany). IND (Mw = 357.8 g/mol) was obtained from Fagron A/S (Copenhagen, Denmark). Ethanol (Ph. Eur. grade) was obtained from VWR (Herlev, Denmark). Fasted state simulated intestinal fluid (FaSSIF v1) powder was purchased from Biorelevant Ltd. (London, United Kingdom).
Keyoomars Khorami, Anette Müllertz, Thomas Rades, Influence of preparation method and choice of phospholipid on co-amorphization, physical stability, and dissolution behavior of equimolar indomethacin-phospholipid systems – A case study, Journal of Drug Delivery Science and Technology, Volume 93, 2024, 105433, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105433.

