Characterization and Exploration of Placket–Burman-Designed Porous Calcium Carbonate (Vaterite) Microparticles
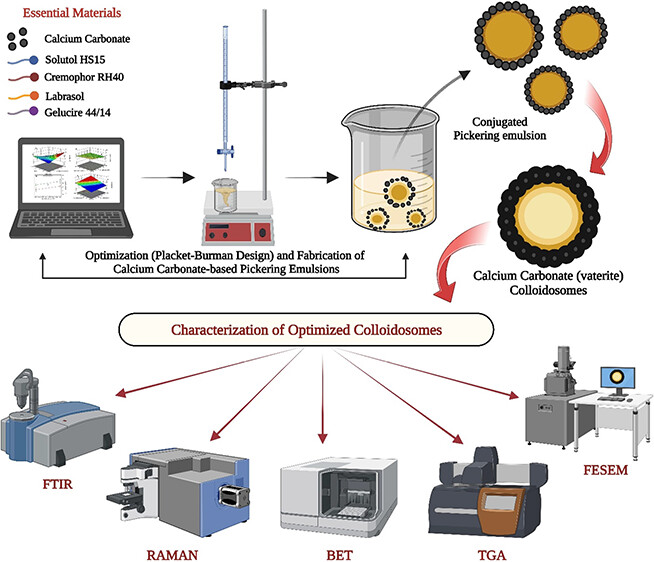
The objective of the research was to identify significant variables that impact the porosity-related properties of CaCO3 particles. The Placket–Burman design was employed to screen multiple variables, including pH, molar concentrations of calcium chloride and sodium carbonate, temperature, concentration of Gelucire 44/14, Cremophor RH40, Solutol HS15, Labrasol, mixing rate, reaction time, and order of addition. The response variables were surface area, pore radius, and pore volume. Influential methodologies such as XRD, FTIR, Raman spectroscopy, and TGA were utilized to validate the precipitate type. The BET surface area ranged from 1.5 to 16.14 m2/g, while the pore radius varied from 2.62 to 6.68 nm, and the pore volume exhibited a range of 2.43 to 37.97 cc/gm. Vaterite structures with spherical mesoporous characteristics were observed at high pH, whereas calcite formations occurred at low pH. The order of addition impacted the surface area but did not affect the pore volume. To maximize the surface area, a lower reaction time and molar concentrations of sodium carbonate were found to be advantageous. The pore radius was influenced by the pH, surfactants, and reaction conditions. The sediments were categorized based on the percentage of vaterite formation. The instrumental techniques effectively characterized the precipitates and provided a valuable complementary analysis.
1. Introduction
2.1. Materials
Calcium chloride (CaCl2) and sodium carbonate (Na2CO3) were acquired from Sigma Aldrich (India) for this study. Additionally, Solutol HS15 and Cremophor RH40 were generously provided as gift samples by BASF (USA), while Labrasol and Gelucire 44/14 were supplied ex gratia by Gattefosse (France). A mechanical stirrer (IKA RW 20 digital, India) was used to mix the contents. GPT-4 and Grammarly served as AI-assistants in drafting the final manuscript.
Download the full study as PDF here: Characterization and Exploration of Placket–Burman-Designed Porous Calcium Carbonate (Vaterite) Microparticles
or read it here
Avi Singh, Sabya Sachi Das, Priya Ranjan Prasad Verma, Janne Ruokolainen, Kavindra Kumar Kesari, and Sandeep Kumar Singh. Characterization and Exploration of Placket–Burman-Designed Porous Calcium Carbonate (Vaterite) Microparticles. ACS Omega Article ASAP.

