Synthesis and Evaluation of Novel Functional Polymers Derived from Renewable Jasmine Lactone for Stimuli-Responsive Drug Delivery
Abstract
Instances of synthetic polymers obtained from renewable feedstock with the possibility of post-synthesis functionalization are scarce. Herein, the first ever synthesis and drug delivery application of amphiphilic block copolymer (mPEG-b-PJL) derived from renewable jasmine lactone with free allyl groups on the backbone is presented. The polymer is synthesized via facile ring-opening polymerization and subsequently, UV mediated thiol-ene click chemistry is utilized for post-functionalization. The introduction of hydroxyl, carboxyl, and amine functionality to mPEG-b-PJL polymer is successfully established. As a proof-of-concept demonstration, doxorubicin (DOX) is conjugated on hydroxyl-terminated polymer (mPEG-b-PJL-OH) via redox responsive disulfide linkage to obtain PJL-DOX. PJL-DOX is readily self-assembled into micelles with an average hydrodynamic size of ≈150 nm and demonstrates reduction-responsive DOX release. Micelles are evaluated in vitro for cytocompatibility and selective drug release in cancer cells (MDA-MB-231) using 10 mm glutathione as a reducing agent. Cytotoxicity and microscopy results confirm a redox-triggered release of DOX, which is further confirmed by flow cytometry. The introduction of these novel functional polymers can pave the way forward in designing polymer-drug conjugate-based smart nano-carriers.
Introduction
Polymers derived from renewable feedstock have gained prominent attention in recent years as a sustainable alternative to fossil-based materials due to the depletion of fossil fuels and government policies, which are aiming to promote greener solutions.[1] Polymers and more specifically, polyesters have gained a significant position in drug delivery mainly owed to their well-controlled biodegradability. Poly(lactic acid) (PLA), and its copolymers such as poly(lactic-co-glycolic acid) (PLGA), are the ideal examples of polyesters derived from renewable feedstock that can further be produced via facile ring-opening polymerization (ROP) with full control on polymer structure. Nevertheless, poor drug loading is often observed with PLA-based materials owing to its low hydrophobicity and thus, more hydrophobic versions of PLA have been prepared to increase the stability and hydrophobicity of PLA-based drug delivery carriers.[2] However, synthesis of these alkyl-substituted lactides seems tedious and thus, a straightforward methodology of preparing amphiphilic block copolymer using renewable poly(decalactone) (PDL) was recently reported. PDL-based drug delivery carriers were evaluated largely for their toxicity, biodegradability, and their potential as drug delivery carriers.[3-6]
Since PDL is an amorphous polymer, PDL-based micelles demonstrate a rapid drug release along with initial burst release. In the absence of control over drug release, premature drug leakage is usually observed from micelles during storage and/or after administration in the human body (before reaching the actual target site), which could lead to suboptimal therapeutic activity.[7] In recent years, polymer-drug conjugates (PDCs) have emerged as an effective approach to circumvent the problems associated with premature drug release and to improve the overall efficiency of therapy. In addition, conjugation of polymers to the therapeutics offers improved pharmacokinetic and pharmacodynamic properties such as increased plasma half-life, protection of the drug/bioactives degradation from degrading enzymes, enhanced stability of proteins, and enhanced solubility of low-molecular-weight drugs.[8] Moreover, drug conjugation via stimuli-responsive linkers affords additional control over the drug release at selective target sites.[9]
The availability of free functional groups on the polymer backbone is a crucial pre-requisite for the development of efficient PDCs. Several reports have been published discussing the approaches for preparing functional polyesters[10] but literatures presenting their drug delivery applications are rare, owing to the complex design and limited functionality. For instance, poly(glutamic acid) (PGA) was utilized for conjugation of camptothecin, and the resultant product (CT-2106) entered clinical trials. However, the polymer PGA is limited to drug molecules that have free alcohol groups for conjugation. Moreover, the reaction conditions require protection and de-protection steps and thus, is not an industrial friendly approach.[11] Another successful synthetic polymer used for fabricating PDCs to date is poly(ethylene glycol) (PEG), and approximately twelve such formulations are available on the market.[8] However, non-degradability, poor drug loading due to lack of functional groups (type and number), and PEG immunogenicity limit its applications. Consequently, some PEG-based products were withdrawn from the market.[12]
A simplistic and reproducible design of biodegradable polymer with the scope of excellent tunability as per desired drug delivery application using economical/renewable feedstock is still the major key point for successful translation of polymer-based drug delivery systems from bench to bedside. Monomers containing free “ene” groups in their backbone are of immense interest for polymer synthesis due to the possibilities it offers to insert functional groups of interest via facile thiol-ene click reaction. Moreover, the “ene” group does not interfere in the ROP reactions, which is one of the most acceptable and reliable reaction mechanisms to produce high molecular-weight polyesters with precise chain length. The ROP of functional renewable monomer α-methylene-γ-butyrolactone (MBL) is one of the best examples, which was utilized to generate polymers with “ene” handles.[13] However, specific catalysts and stringent reaction conditions are required to acquire polymers using MBL, which restrict its widespread applications and are apparent from the lack of published reports presenting the applications of poly(MBL).
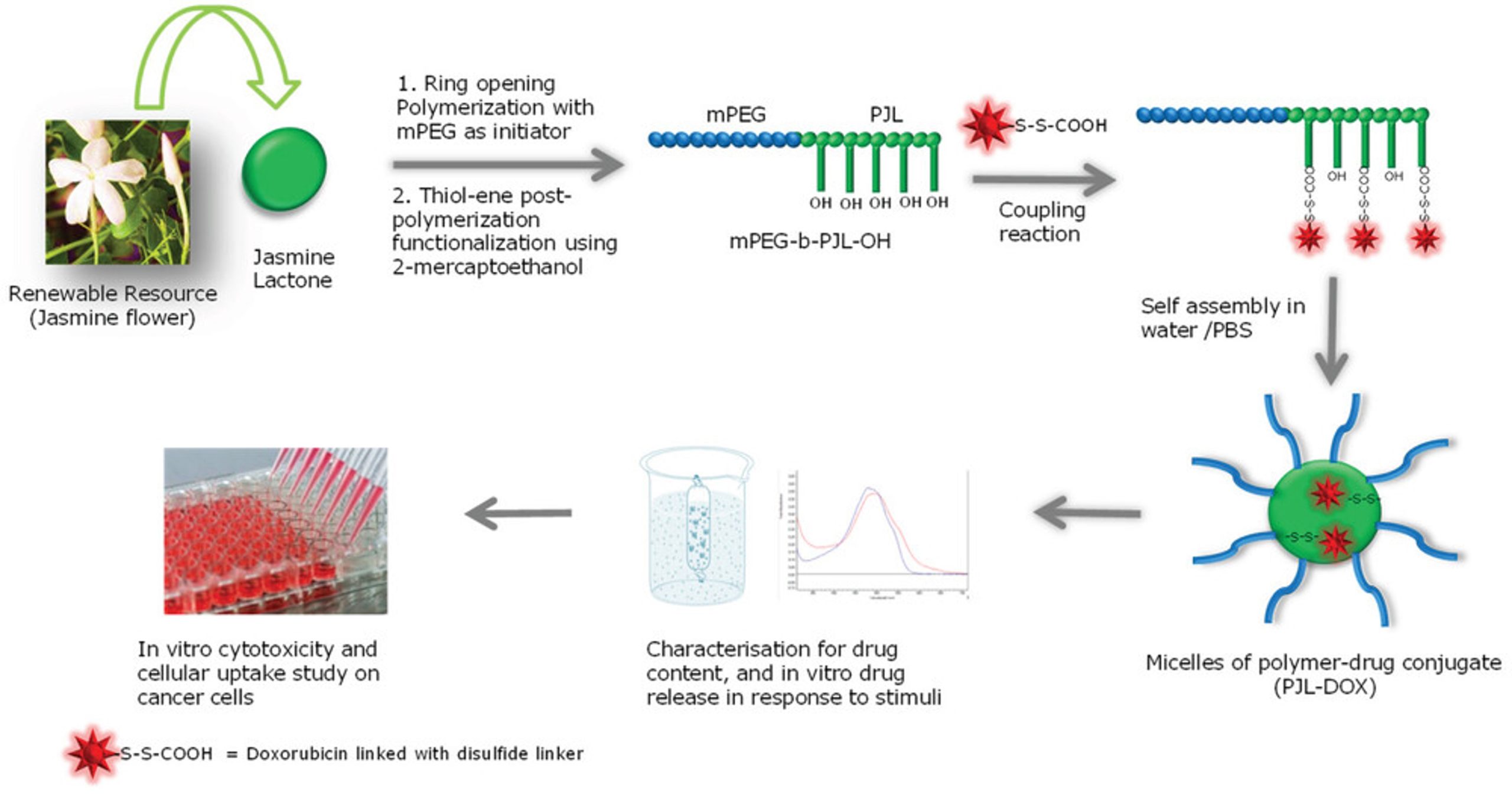
The polymer science domain is still looking for a polymer, which can be synthesized easily with ample possibilities of post functionalization as per the desired requirement. Therefore, here, we are presenting the synthesis, characterization, and one of the several possible applications of a novel polymer, called poly(jasmine lactone) (PJL) with functional handles. Subsequently, we have designed a PJL-drug conjugate using doxorubicin (DOX) as a drug, with disulfide bond as a reduction sensitive linker to demonstrate the selective drug release capability in the presence of glutathione (GSH) (Figure 1). It is widely reported that disulfide linker cleaved in the presence of GSH, which is usually found in high levels within cancer cells compared to normal cells.[14, 15] Thus, selective drug release in cancer treatment could be highly beneficial considering the serious side effects of chemotherapy.
The polymer is produced via a facile methodology using renewable monomer (jasmine lactone) with generous modification possibilities. Jasmine lactone is a United States Food and Drug Administration approved food additive and a Generally Recognized As Safe (GRAS) substance, found in several natural substances including jasmine oil, tea, lily, ginger, peach, etc. This monomer provides two unique properties: 1) polymer synthesis in a highly controlled fashion via well-established ROP technique and 2) possibility of post-polymerization functionalization via facile thiol-ene click reaction without additional efforts, such as protection-deprotection. To the best of our knowledge, this is the very first report discussing the synthesis, post-functionalization, and drug delivery capability of PJL.
Download the full article as PDF here Synthesis and Evaluation of Novel Functional Polymers Derived from Renewable Jasmine Lactone for Stimuli-Responsive Drug Delivery
or read more here
Synthesis and Evaluation of Novel Functional Polymers Derived from Renewable Jasmine Lactone for Stimuli-Responsive Drug Delivery, Kuldeep Kumar Bansal, Ezgi Özliseli, Ari Rosling, Jessica Marianne Rosenholm, First published: 14 June 2021, https://doi.org/10.1002/adfm.202101998

