Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma
The anti-inflammatory drug ketoprofen has shown promising results in the field of drug repurposing for the treatment of brain cancer, but currently developed formulations are for invasive administration (intravenous) and have very limited drug strength. Hence, the purpose of this work was to develop an intranasal oil-in-water nanoemulgel, with drug strength maximization, for noninvasive, more effective and safer treatment of glioma. The developed formulations were made of Capryol® 90 (hydrophobic surfactant), Tween® 80 (hydrophilic surfactant), Transcutol® (co-solvent and permeation enhancer), Pluronic® F-127 (surfactant and gelling agent) and ketoprofen.
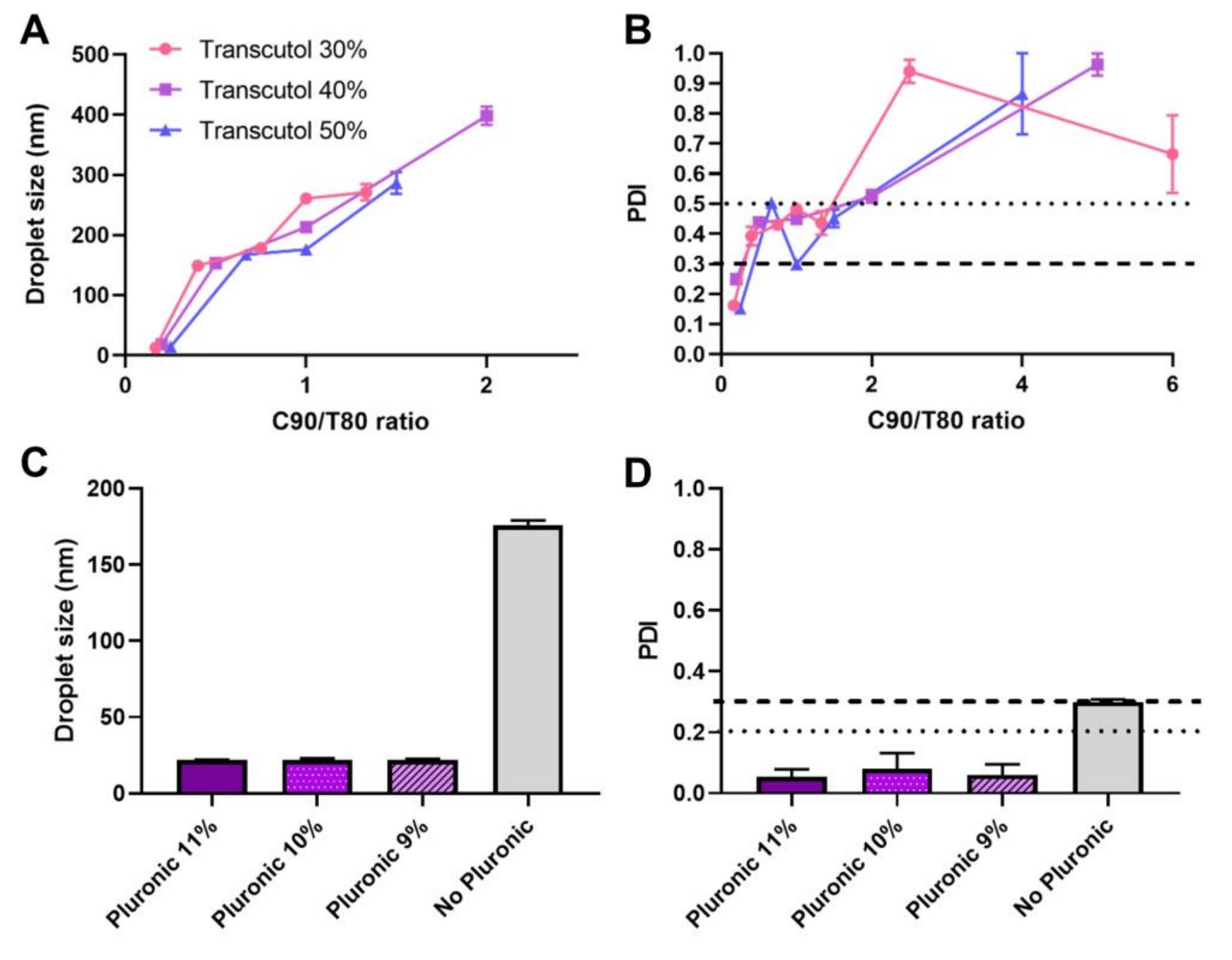
Droplet size, polydispersity index, in vitro drug release and accelerated stability were measured. Results showed that the addition of Pluronic to a preliminary optimized nanoemulsion led to a significant droplet size and PDI reduction (176 to 22 nm, and 0.3 to 0.1, respectively). Achieved drug strength was 4 mg/mL, which is more than 50 times higher than ketoprofen’s aqueous solubility. The developed formulations also appeared to have high stability, with instability indexes between 0.130 and 0.265, and high cumulative drug release percentage, varying between 78 to 93% after 24h.
Formulations also showed a controlled release profile, fitting a Korsmeyer-Peppas kinetic model, with low AIC (43.84 to 54.67) and high R2 (0.9725 to 0.9971) values, depicting non-Fickian diffusion (n between 0.7 and 0.8). Hence, high drug strength, high stability and high drug release ketoprofen-loaded nanoemulgels were successfully prepared. Future in vitro cytotoxicity evaluation in glioma cells will assess the true potential of the developed formulations for the treatment of brain cancer.
Download the full article as PDF here Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma
Material
Ketoprofen, Tween® 80 (polysorbate 80) and Pluronic® F-127 (poloxamer 407) were purchased from Sigma-Aldrich (Steinheim, Germany). Capryol® 90 (propylene glycol monocaprylate, type II) and Transcutol® (diethylene glycol monoethyl ether) were gift samples from Gattefossé (Lyon, France). The preliminary oil-in-water nanoemulsions were prepared by spontaneous emulsification. The first step was adding and mixing different ratios of Capryol 90 (oil and hydrophobic surfactant), Tween 80 (hydrophilic surfactant), and Transcutol (co-solvent, cosurfactant and permeation enhancer), with ratios varying from 1 to 6 parts Capryol, 1 to 6 parts Tween, and 3 to 5 parts Transcutol, to make a preconcentrate. After this, the drug was solubilized in the prepared preconcentrate, and only after was the water added and mixed, giving origin to preliminary nanoemulsions. Lead nanoemulsions were transformed into nanoemulgels by adding a Pluronic® F-127 (surfactant and gelling agent) aqueous solution to the preconcentrate, instead of water.
Pires, P.C.; Correia, M.; Veiga, F.; Paiva-Santos, A.C. Anti-Inflammatory Drug Repurposing for Intranasal Delivery: Ketoprofen Nanoemulgel Development for the Treatment of Glioma. Eng. Proc. 2023, 52
Read more on Orally Disintegrating Tablets (ODTs) here:


