A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives
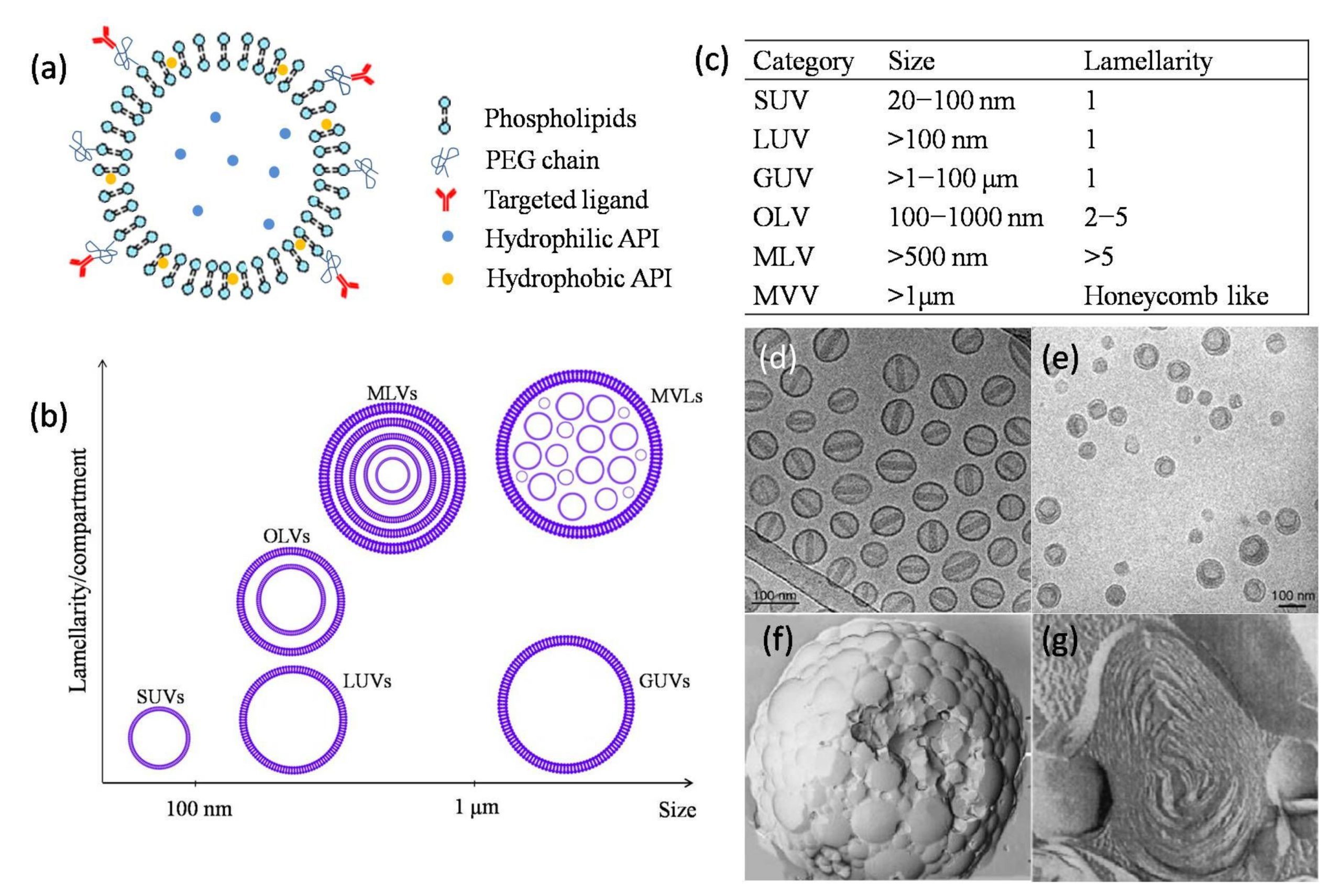
Liposomes have been considered promising and versatile drug vesicles. Compared with traditional drug delivery systems, liposomes exhibit better properties, including site-targeting, sustained or controlled release, protection of drugs from degradation and clearance, superior therapeutic effects, and lower toxic side effects. Given these merits, several liposomal drug products have been successfully approved and used in clinics over the last couple of decades.
In this review, the liposomal drug products approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) are discussed. Based on the published approval package in the FDA and European public assessment report (EPAR) in EMA, the critical chemistry information and mature pharmaceutical technologies applied in the marketed liposomal products, including the lipid excipient, manufacturing methods, nanosizing technique, drug loading methods, as well as critical quality attributions (CQAs) of products, are introduced.
Additionally, the current regulatory guidance and future perspectives related to liposomal products are summarized. This knowledge can be used for research and development of the liposomal drug candidates under various pipelines, including the laboratory bench, pilot plant, and commercial manufacturing.
Download the full article as a PDF here or read it here
Article information: Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. https://doi.org/10.3390/molecules27041372

