Self-Emulsifying Drug Delivery System for Enhanced Oral Delivery of Tenofovir: Formulation, Physicochemical Characterization, and Bioavailability Assessment
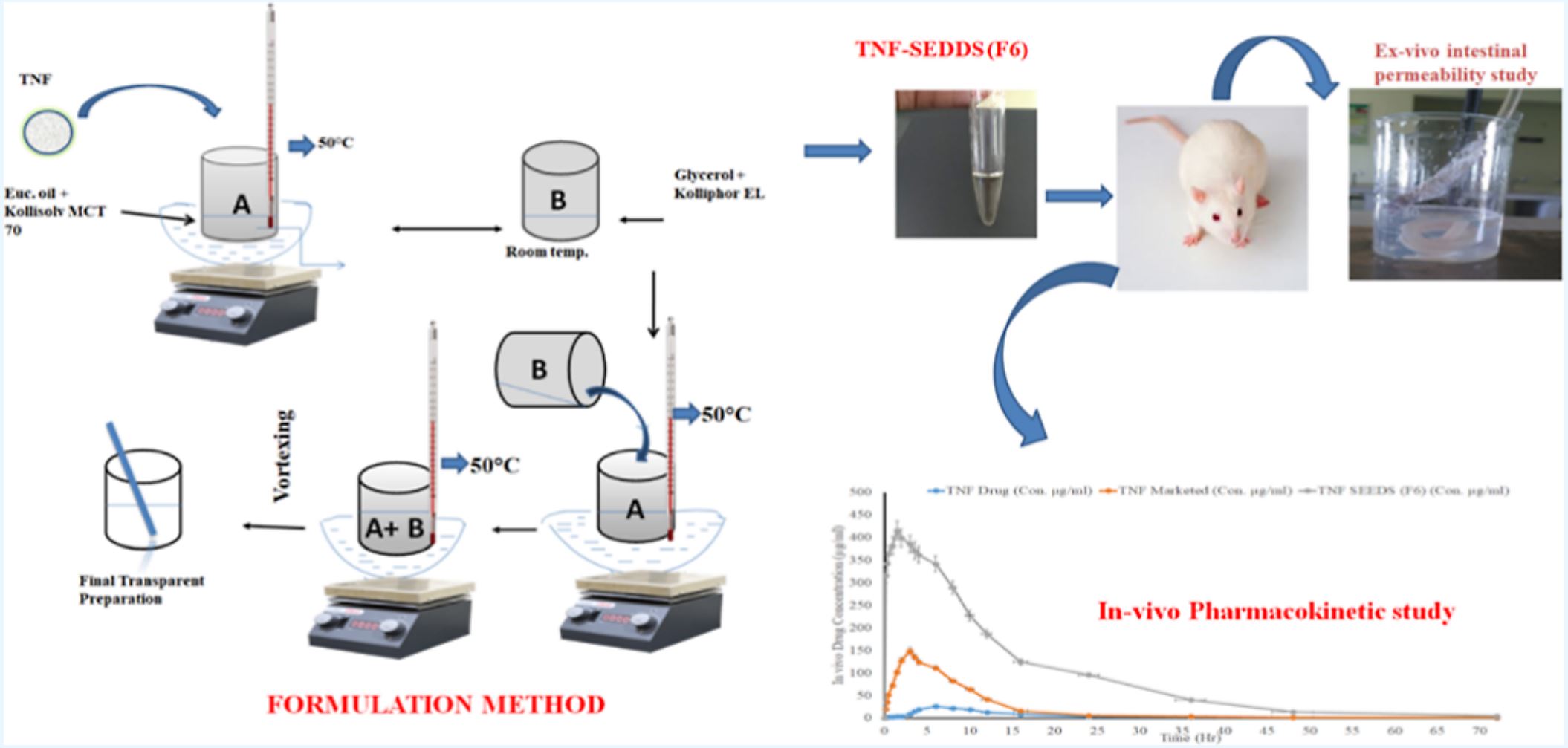
Tenofovir (TNF) is a common component of many antiretroviral therapy regimens, but it is associated with poor membrane permeability and low oral bioavailability. To improve its oral bioavailability and membrane permeability, a self-emulsifying drug delivery system (SEDDS) was developed and characterized, and its relative bioavailability was compared to the marketed tablets (Tenof). Based on solubility and ternary phase diagram analysis, eucalyptus oil was selected as an oil phase, Kolliphor EL, and Kollisolv MCT 70 were chosen as surfactant and cosurfactant, respectively, while glycerol was used as cosolvent in surfactant mixture.
Optimized SEDDS formulation F6 showed an oil droplet size of 98.82 nm and zeta potential of −13.03 mV, indicating the high stability of oil droplets. Differential scanning calorimetry, X-ray diffraction, and scanning electron microscopy characterization studies were also carried out to assess the amorphous and morphological states of the drug in the prepared SEDDS formulation. The in vitro dissolution profile of SEDDS shows the rapid release of the drug. SEDDS F6 demonstrates a higher drug permeability than the plain TNF and TNF-marketed tablets (Tenof). A pharmacokinetic study in rats revealed that SEDDS F6 showed significantly higher Cmax and AUC0–t than the marketed tablets and pure drug suspension.
In addition, the relative bioavailability of SEDDS formulation dramatically improved by 21.53-fold compared to marketed tablets and 66.27-fold compared to pure drugs. These findings show that SEDDS composed of eucalyptus oil, glycerol, Kolliphor EL, and Kollisolv MCT 70 could be a useful tool for enhancing physiochemical properties and oral TNF absorption. Therefore, SEDDS has shown promise in improving the oral bioavailability of poorly water-soluble drugs.
Download the full article as PDF here Self-Emulsifying Drug Delivery System for Enhanced Oral Delivery of Tenofovir
read more here
Chemicals and Reagents
Emcure laboratories. Pune, India, provided TNF as a free sample. Kolliphor EL (polyoxyl castor oil), Kollisolv MCT 70 (medium chain triglycerides), and Kolliphor HS15 (polyoxyl 15 hydroxystearate) were complementary samples provided by BASF, Mumbai, India. Raj Chemicals provided glycerol. Propylene glycol and caprylic acid were purchased from Loba Chemicals, Mumbai, India. The analytical grade was used for all other chemicals.
Self-Emulsifying Drug Delivery System for Enhanced Oral Delivery of Tenofovir: Formulation, Physicochemical Characterization, and Bioavailability Assessment, Nilesh Mahajan, Md Ali Mujtaba, Ritesh Fule, Sonali Thakre, Md Sayeed Akhtar, Sirajudeen S. Alavudeen, Md Khalid Anwer, Mohammed F. Aldawsari, Danish Mahmood and Md Sarfaraz Alam, https://doi.org/10.1021/acsomega.3c08565
Read more on Orally Disintegrating Tablets (ODTs) here:


