Sensitivity analysis of the formation of hollow solid lipid micro- and nanoparticles from CO2-saturated solution of fully hydrogenated soybean oil
Abstract
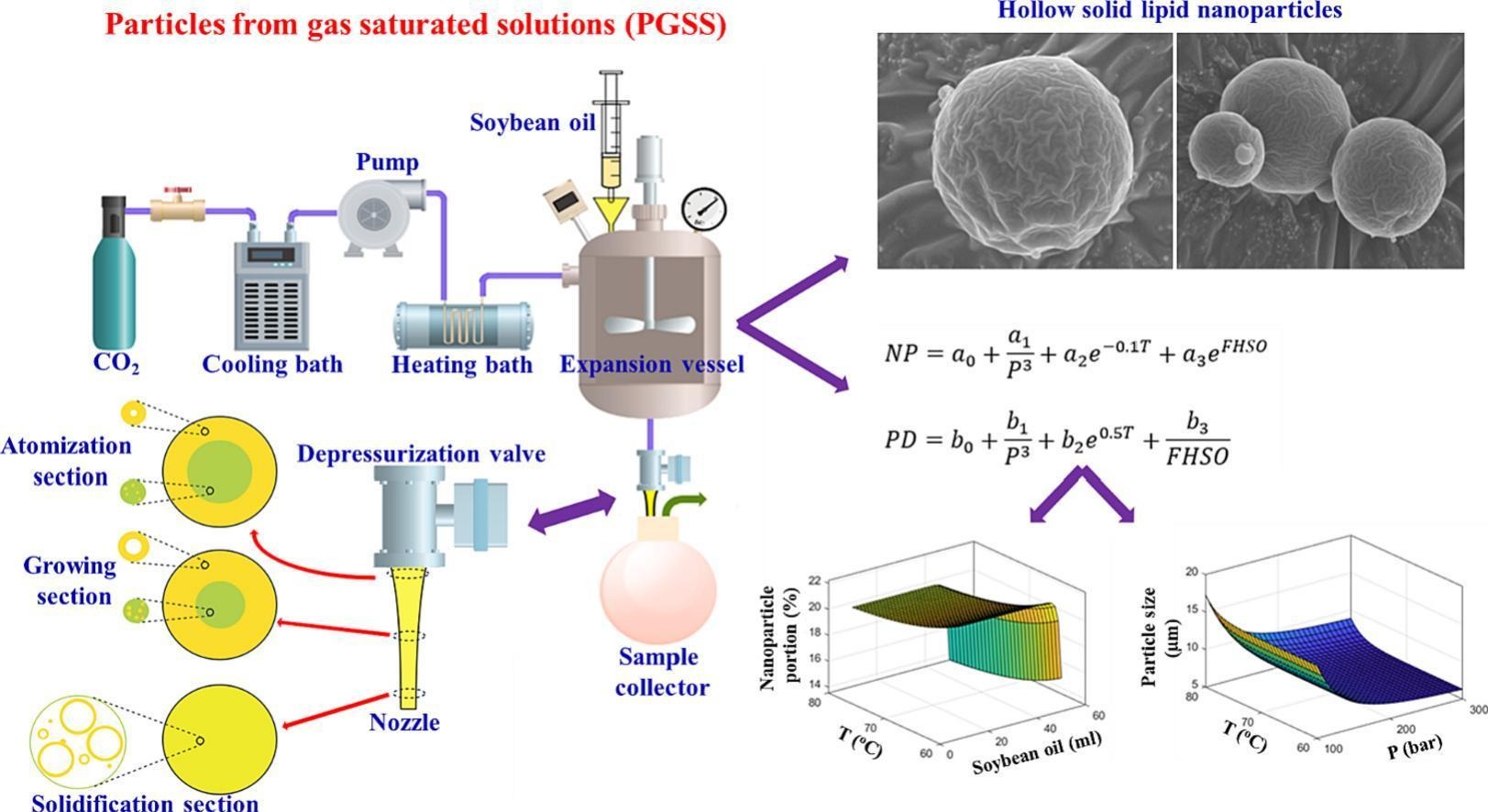
The production of hollow solid micro- and nanoparticles using fully hydrogenated soybean oil (FHSO) and supercritical carbon dioxide (SC-CO2) has been studied in the literature. This research aimed to examine the impact of FHSO volumes (10 to 50 ml), temperatures (60 to 80 °C), and pressures (100 to 300 bar) on particle size distribution, morphology, and melting properties of FHSO hollow solid lipid micro- and nanoparticles. The experimental results were extensively interpreted using principles from thermodynamics and hydrodynamics. Two equations were developed to relate experimental data of particle diameter (PD) and nanoparticle portion (NP) to pressure, temperature, and FHSO volume. Optimum conditions for minimizing PD and maximizing NP were identified at a pressure of 300 bar, a temperature of 60 °C, and an FHSO volume of 20 to 30 ml. Under these conditions, the PD ranged from 6.3 to 6.6 μm, and the NP reached 24.1%.
Introduction
Solid lipid nanoparticles have recently emerged as a promising approach for delivering lipophilic drugs and bioactive compounds, offering several advantages including biocompatibility, improved protection of the active ingredient within the solid lipid matrix, and controlled release [1,2]. However, certain limitations have been identified, such as the limited loading capacity resulting from the solid core structure and the occurrence of burst release during solid lipid core crystallization [3]. Additionally, conventional production methods for solid lipid nanoparticles have their own drawbacks, such as the use of solvents in certain solvent emulsification/evaporation techniques, the potential instability of bioactives in hot homogenization and high shear homogenization methods, and the risk of metal contamination in ultrasonication processes [4,5].
Supercritical fluid (SCF)-based techniques present viable solutions to address the limitations commonly encountered in conventional methods for micro- and nanoparticle production. These SCF-based techniques leverage the unique properties of supercritical fluids, which exhibit liquid-like density and solubility, as well as gas-like viscosity and diffusivity, resulting in a substantial enhancement of the mass transfer rate [6,7]. Among SCFs, carbon dioxide is the most commonly employed due to its low critical pressure and temperature, cost-effectiveness, non-toxicity, non-flammability, and its complete separation from the final product through depressurization [8,9]. Rapid expansion of supercritical solution (RESS) [10,11] and particles from gas-saturated solutions (PGSS) [12] are two well-established techniques that rely on the utilization of SCFs.
In the RESS process, the solute is initially dissolved in a SCF, and the resulting solution is subsequently depressurized by flowing through a nozzle at high speed. This rapid expansion leads to the crystallization of micro- and nanoparticles [13]. However, one challenge of the RESS method is that many pharmaceutical materials have low solubility in SC-CO2. This necessitates the use of a large quantity of CO2, which can increase equipment size and operational costs. Alternatively, the addition of a polar cosolvent, such as methanol, may be required to enhance solubility. However, the presence of residual cosolvent can contaminate the resulting particles [14]. These limitations are addressed by the PGSS method. In PGSS, the SCF is first dissolved in a melt solution or suspension, rather than directly dissolving the solute in the SCF. The resulting gas-saturated solution is then rapidly depressurized as it passes through a nozzle [15]. The PGSS process consumes a lower amount of dense gas compared to the RESS process due to the higher solubility of gases in liquids relative to the solubility of liquids in gases [16]. In a study conducted by Yang and Ciftci, they successfully produced hollow solid lipid micro- and nanoparticles using the PGSS method [4]. The distinctive structure and geometry of these solid lipid particles have positioned them as a promising platform for drug loading and delivery.
The solid lipid shell provides effective protection for the loaded drugs and food, while the hollow structure significantly enhances the loading capacity. Compared to liquid formulations of solid lipid nanoparticles, the dry free-flowing products offer advantages in terms of ease of handling and storage, and the simplified and clean procedure facilitates scaling up [4]. Peppermint essential oil [17] and fish oil [18] were successfully loaded into hollow solid lipids made from fully hydrogenated soybean oil (FHSO). The loading efficiencies achieved were 47.5% for peppermint essential oil and 97.5% for fish oil, both at an initial oil concentration of 50% and under the following particle formation conditions: 200 bar expansion pressure, 57 °C expansion temperature, and 50 μm nozzle diameter. These specific conditions were determined based on the optimization process conducted in our previous study, which aimed to achieve the smallest hollow solid lipid micro- and nanoparticles [4].
A detailed examination of the study conducted by Yang and Ciftci [4] reveals that their optimization of the process focused solely on the effects of pressure and nozzle diameter while keeping temperature and FHSO volume constant. However, a comprehensive review of the literature indicates that the performance of processes involved in the formation of particles from gas-saturated mixtures is influenced not only by pressure and nozzle size but also by temperature and the initial amount of substance. In a study conducted by Yun et al. [19], the PGSS method was employed to produce microparticles of lecithin with polyethylene glycol (PEG). The effects of expansion temperature (40–50 °C), pressure (200–300 bar), nozzle diameter (250–300 μm), and stirring speed (200–400 rpm) on both particle size and lecithin loading in PEG were investigated. The particle size varied between 0.74 and 1.62 mm, with smaller particles obtained at lower temperatures, higher pressure, smaller nozzle diameter, and higher stirring speed. Additionally, the study demonstrated that the amount of entrapped lecithin in PEG increased with the supercritical phase density. In a different study conducted by Gil’mutdinov et al. [20], the synthesis of ibuprofen/PEG 4000 and methylparaben/PEG 4000 nanoparticles using the PGSS technique was examined. The study evaluated the effects of varying pressures (100–300 bar), temperatures (40–80 °C), and nozzle diameters (200–500 μm) on the particle size of the composites. The results revealed that increasing pressure and reducing nozzle diameter led to a decrease in particle size for both composites. On the other hand, temperature had contrasting effects: it increased the mean size of ibuprofen/PEG 4000 nanoparticles while decreasing the mean size of methylparaben/PEG 4000 nanoparticles.
Accordingly, the primary objective of this study was to enhance our previous research by optimizing the PGSS technique for the production of FHSO hollow solid lipid micro- and nanoparticles. In order to achieve this goal, we conducted experimental investigations to assess the influence of temperature, pressure, and FHSO volume on the generation of hollow solid lipid micro- and nanoparticles through the atomization of a SC-CO2-expanded molten FHSO. Our optimization efforts were focused on attaining the lowest possible average particle diameter (PD) and the highest achievable nanoparticle portion (NP) in the final product. Once the optimal processing parameters for the production of FHSO hollow solid lipid micro- and nanoparticles using the PGSS method are determined, it opens up the opportunity to enhance the loading performance of essential oils and other pharmaceuticals into these particles.
Read more here
Tahmasb Hatami, Junsi Yang, M. Angela A. Meireles, Ozan N. Ciftci, Sensitivity analysis of the formation of hollow solid lipid micro- and nanoparticles from CO2-saturated solution of fully hydrogenated soybean oil, Powder Technology, 2023, 119189, ISSN 0032-5910, https://doi.org/10.1016/j.powtec.2023.119189.
Read more on “Pharmaceutical Oils” here:


