Sex-Based Differences in the Biodistribution of Nanoparticles and Their Effect on Hormonal, Immune, and Metabolic Function
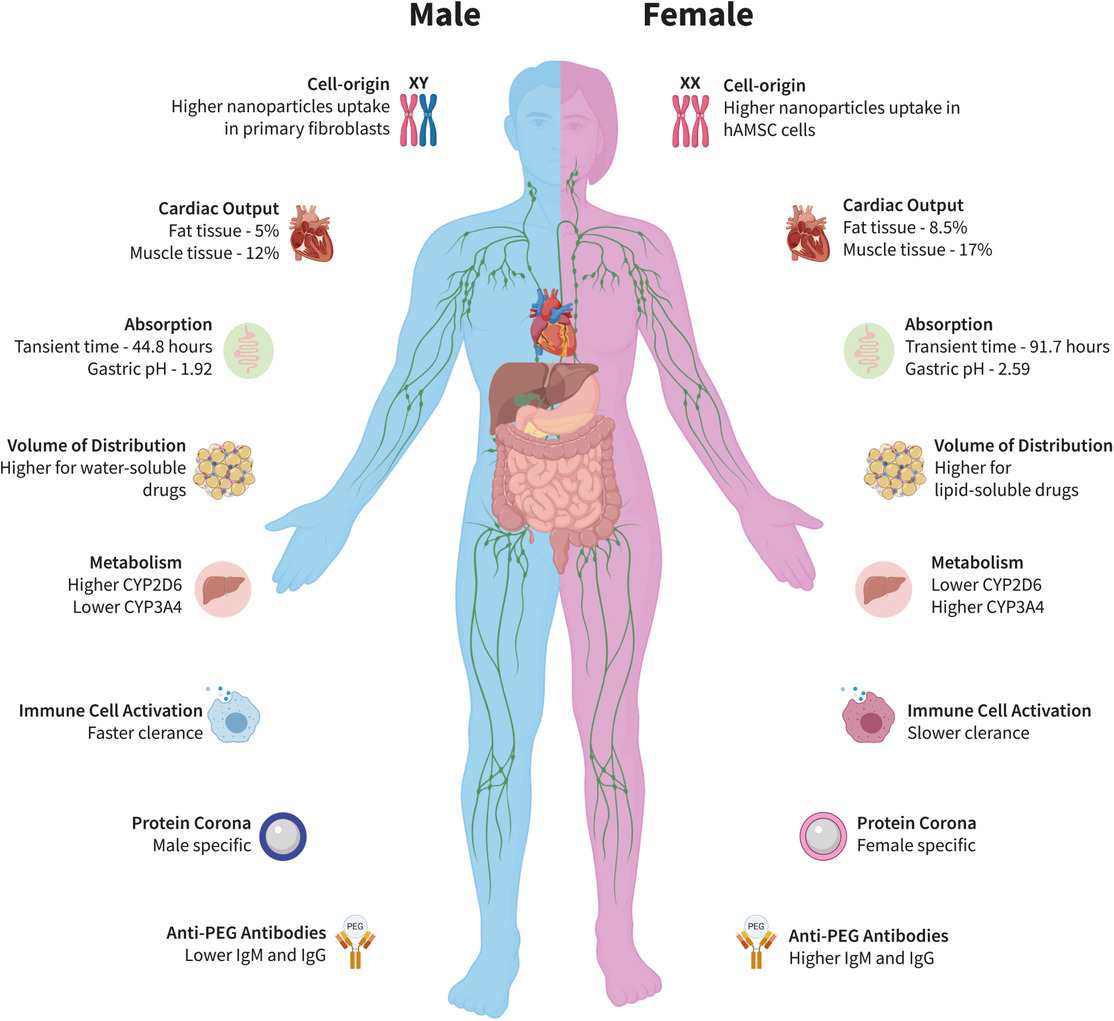
Males and females respond differently to medications due to physiologic, metabolic, and genetic factors. At times, sex-related differences cannot be mitigated by dose adjustment to body mass, and are evident from the tissue level to the single cell. The rising number of clinically approved nanotechnologies calls for assessing how their activity is affected by the patient’s sex. Herein, sex differences in nanotechnology are scoped, with emphasis on molecular considerations. Sex-specific pharmacokinetics of nanocarriers is influenced by the nanoparticle’s composition, its size, and architecture. The biodistribution and immune response to nanoparticles in males and females, and the influence nanoparticles have on hormones, fertility, and toxicity, are discussed. Despite its importance, the effect of sex on the design and implementation of nanomedicines is underresearched. Herein, it is aimed to raise awareness of sex differences in the preclinical and clinical evaluation of nanotechnologies.
Introduction
Sex-related response to some medications is associated with genetic, anatomical, and molecular differences between females and males.[ 1 ] New nanotechnology applications in medicine[ 2 ] have added another level of complexity in assessing how sex affects nanoscale drugs in the body. Despite the increased awareness for including sex as a biological variable, the influence of sex on nanotechnologies’ fate in the body is not well understood.[ 3 ] From a historical perspective, only in 1993 the US Food and Drug Administration (FDA) lifted restrictions that excluded females from participating in most clinical trials.[ 4 ] As a result, it was found that some drugs affect females differently than males, at times, resulting in severe adverse effects seen primarily in females.[ 5 ] This was followed by changing the dosing regimens, or even withdrawal, of drugs, which imposed risks to females’ health.[ 6 ] For example, the antihistamine terfenadine and the drug cisapride monohydrate, which increases gastrointestinal contractions, were both found to cause potentially fatal arrhythmias in females.[ 7 ] This prompted the study of how physiological differences between males and females impact pharmacological responses.[ 8 ] In 2013, the doses of zolpidem-based products were lowered for females, but not for males, following unexplained car accidents involving female drivers that fell asleep at the wheel.[ 9 ] Although sex-related differences encouraged a change in drug dosing between sexes, they were mainly addressed by merely adjusting the dose according to the patient’s body mass or body surface area.[ 10 ] However, not all sex differences can be addressed in this manner. More specifically, hormonal, genetic, immunological, and other molecular differences also govern sex-specific responses and pharmacokinetics (PK) profiles.[ 11 ]
Sex differences also affect the biodistribution and clearance of nanoparticle drugs (Figure 1 , Table 1 ). Generally, drug delivery using nanocarriers can alter the PK profile,[ 12 ] lower drug toxicities,[ 13 ] protect delicate biological cargos, and prolong circulation time, thereby expanding the therapeutic window.[ 14 ] The biodistribution and PK of nanoparticles in the body are primarily affected by the nanoparticle’s properties (composition, size, charge, surface modifications, and stability), rather than the loaded drug.[ 12, 15 ] Nonetheless, sex plays an essential physiological role in influencing nanoparticles’ fate in the body as well. A recent article published by our group reveals that the ovulation cycle affects nanoparticles’ accumulation in the female reproductive system.[ 16 ] Furthermore, there are several indications for sex differences in the literature, for example, liposomes loaded with topoisomerase-I inhibitor (CKD602), or topotecan, for cancer treatment of neuroblastoma and carcinoma, had slower clearance rates in female rats compared to male rats, postintravenous (i.v.) administration.[ 17 ] Similarly, the administration of liposomal doxorubicin resulted in slower blood clearance rates in female rats and in humans compared to males.[ 18 ]
Alternatively, nanotechnology can help mitigate sex differences associated with the PK of free drugs. For example, cannabidiol (CBD), delivered orally in its free form, had higher bioavailability in females compared to males.[ 45 ] However, when CBD was delivered using 40–50 nm micelles, composed of vegetable oils and fatty acids, the sex differences were less evident.[ 46 ] Contrarily, although the PK of lipid nanoparticles containing siRNA targeting both vascular endothelial growth factor (VEGF) and kinesin spindle protein (a motor protein involved in the mitosis process) showed similar PK in both sexes, the intravenous administration resulted in higher liver toxicity in male rats compared to female rats.[ 40 ] In this case, sex-related metabolic differences induced difference in toxicity after the siRNA was released from the nanoparticles regardless of the similar PK profile.[ 40 ] The most up-to-date example is the lipid nanomedicine-based COVID19 vaccine. It was reported that females have a stronger immune response which leads to increased vaccine efficacy and that there are sex differences in several adverse reactions.[ 47 ] A proof-of-concept study showed that the sex-dependent response to the vaccine is related to differences in the uptake of the nanoparticles by female and male natural killer (NK) cells.[ 48 ]
Previous reviews in the field have shed some light on how biological sex is considered in clinical trials of nanomedicine and their design,[ 49 ] and provide a detailed comparison between male and female differences at a molecular level.[ 50 ] Here, we address the main findings that are reported in the literature regarding sex differences in nanomedicine and derive conclusions based on pooling all the available data together. We discuss how sex affects the PK and toxicity of nanoparticles (Figure 1, Table 1), key sex-related proteins (Table 2 ), and hormonal differences (Figure 2 , Table 3 ) and main sex-related considerations regarding nanoparticles’ design (Figure 3 , Table 4 ).
Download the full article as PFD here Sex-Based Differences in the Biodistribution of Nanoparticles and Their Effect on Hormonal, Immune, and Metabolic Function
or read it here
Poley, M., Chen, G., Sharf-Pauker, N., Avital, A., Kaduri, M., Sela, M., Raimundo, P.M., Koren, L., Arber, S., Egorov, E., Shainsky, J., Shklover, J. and Schroeder, A. (2022), Sex-Based Differences in the Biodistribution of Nanoparticles and Their Effect on Hormonal, Immune, and Metabolic Function. Adv. NanoBiomed Res. 2200089. https://doi.org/10.1002/anbr.202200089

