Overcoming the Low-Stability Bottleneck in the Clinical Translation of Liposomal Pressurized Metered-Dose Inhalers: A Shell Stabilization Strategy Inspired by Biomineralization
Abstract
Introduction
1.1. Liposomes Act as Superior Drug Delivery Systems
Nanoparticles are highly dispersed supramolecular structures, typically consisting of polymers, with submicron dimensions that are preferably less than 500 nm [1]. Nanoscale materials have wide applications in various fields, including chemistry [2], biology [3], environment [4], and medicine [5], because they possess physical and chemical properties superior to those of bulk materials. Nanoparticles have become a promising drug delivery platform, due to their abilities to improve the stability and solubility of encapsulated goods, promote transmembrane transport, prolong circulation time, enhance safety and efficacy, and overcome the challenges of traditional delivery with untargeted biological distribution [6]. Many nanoparticle-based medicines, or nanomedicines, have been approved by the FDA, like inorganic, polymeric, and lipid-based medicines (Table 1) [7]. The global nanomedicine market was estimated at USD 53 billion in 2009 and was expected to reach USD 100 billion with a booming growth rate of 13.5% [8].
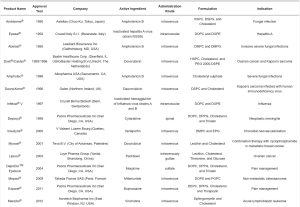
Table 1. FDA-approved nanomedicines reproduced from Mitchell et al. [7] with permission from the Asian Journal of Pharmaceutical Sciences. (click to enlarge)

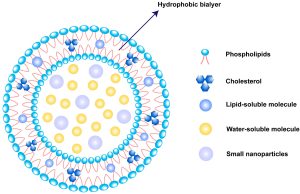
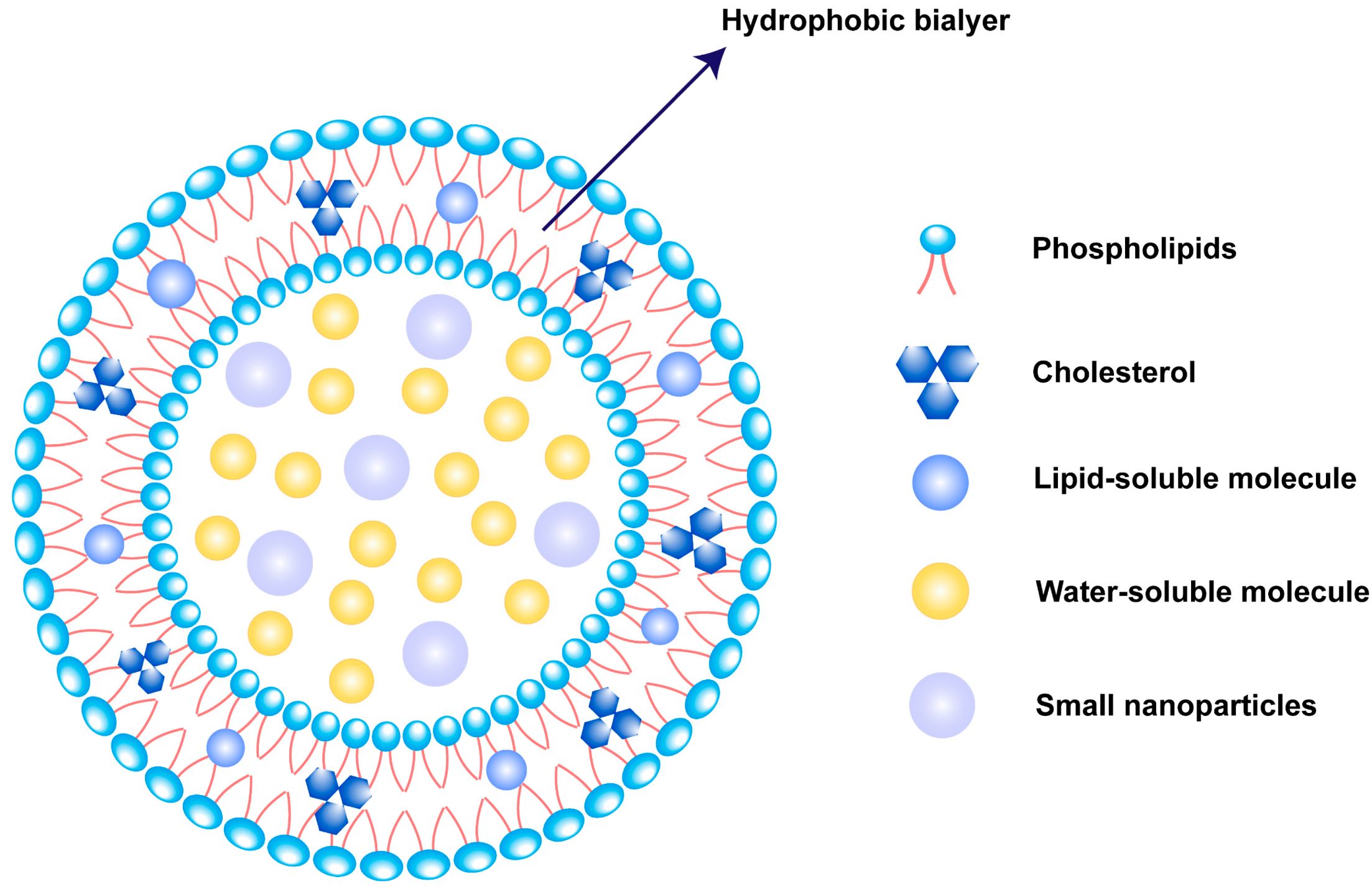
Lipid-based nanoparticles are the most commonly used FDA-approved nanomedicines [9], as shown in Table 1. Outstandingly, liposomes are one of the most widely used lipid-based nanoparticles as a drug delivery platform [10,11].
Part of Table 2. Liposome products on the market, reproduced from He et al. [17] with permission from the Asian Journal of Pharmaceutical Sciences. (click to enlarge)
1.2. Realistic Needs and Advantages of Liposome Pulmonary Delivery
See our next webinar and register here: