Spray coating of cellulose aerogel particles in a miniaturized spouted bed
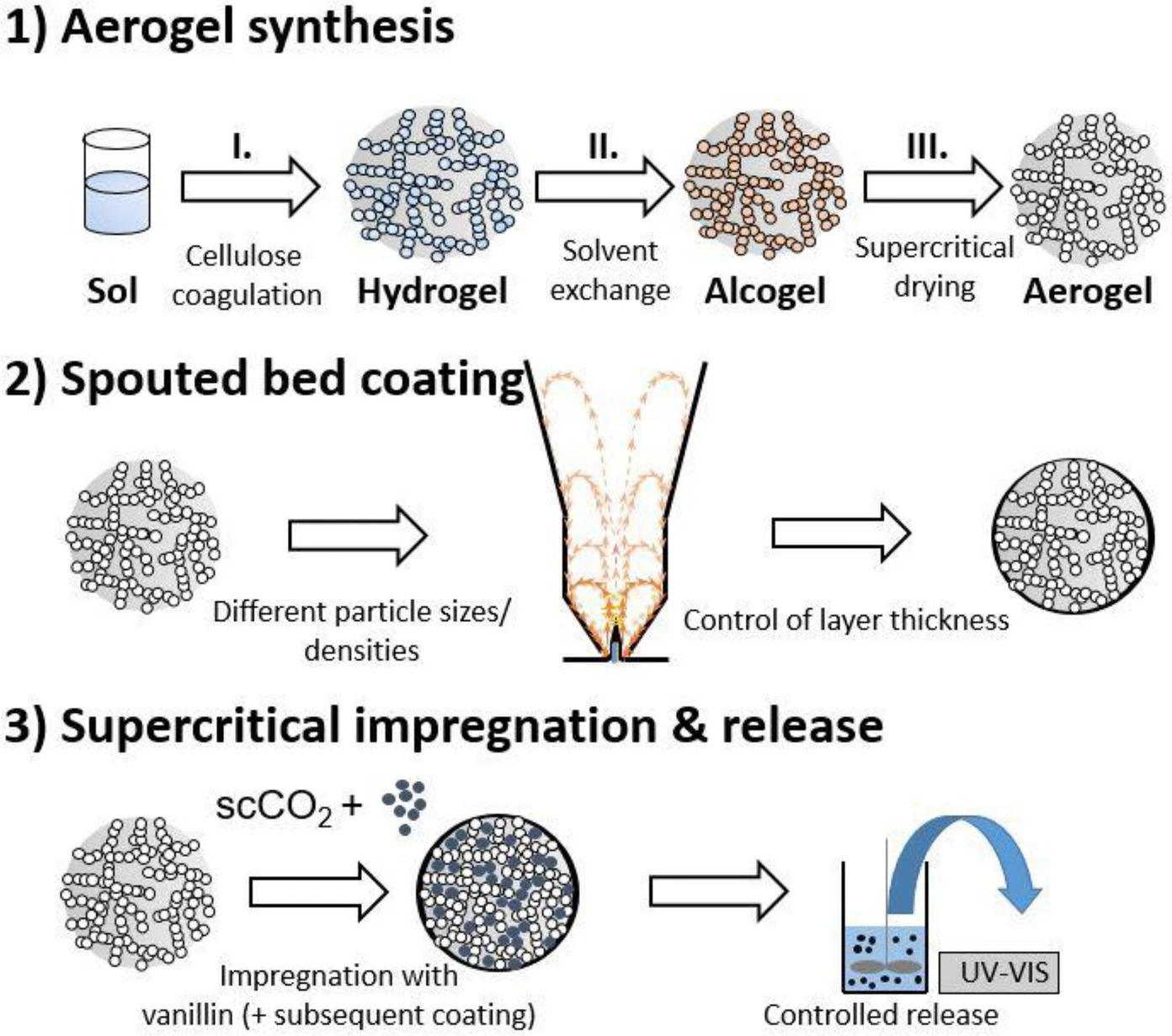
Aim of this work is to apply protective and homogeneous shellac coating layers on the surface of hydrophilic open-pore cellulose aerogel particles with low densities ≤ 0.1 g/cm3 and high specific surface areas in the range of ~ 400–450 m2/g while keeping the aerogels’ microstructure intact during processing. For this purpose, an innovative miniaturized spouted bed setup was used. Successful process settings for application of enclosed films on aerogel surfaces without intrusion of coating material into the pores were determined.
Precise control of coating layer thickness in the range of 10–50 µm was achieved due to variation of coating solution amount without agglomeration and clogging events occurring during processing. Comparison of bulk densities and specific surface areas before and after coating proved the intactness of the porous structure. Coating of particles loaded with vanillin led to controlled release, enhancing release half-life times from 20 to 1600 min. Overall, a successful strategy for coating of organic low-density aerogels was developed.
Download the full article as a PDF here or read it here
Introduction
Aerogels are solid materials with a unique combination of properties such as high porosities, low densities and high specific surface areas (SV). Their synthesis usually involves gelation, solvent exchange and supercritical drying steps (García-González et al. 2011). Besides many other applications, aerogels are suitable as tailorable carrier matrices for flavors and drugs, since they enable the targeted release of the components from the porous structure and offer high loading capacities (Smirnova and Gurikov 2017; Bian et al. 2020). Food- and pharma sectors demand properties such as biodegradability and biocompatibility for in vivo use, which are provided by biopolymeric materials like starch-, alginate- or cellulose-based aerogels which can e.g. be loaded with various active pharmaceutical ingredients (Ulker and Erkey 2014; Veres et al. 2018; Pantić et al. 2016; Mehling et al. 2009; Zeng et al. 2020). The particular suitability of cellulose aerogel matrices as drug delivery systems is rooted in the possibility to induce controlled and hierarchical porosities, their relatively high stability when brought into aqueous environment, high specific surface areas, and the flexibility of macroscopic shape control, which allows the production of spherical particles with defined sizes (Budtova 2019; Volkert et al. 2009; Liebner et al. 2009). Besides, cellulose is a cheap and abundant raw material.
However, some challenges for in vivo use of biopolymer aerogels as carriers arise if a long-term release of the active agent is targeted: due to the hydrophilic surface and the open pore structure of aerogels the penetration of moisture or reactive molecules into the inner part of the materials may cause damage of the pores during storage and lead to aging of encapsulated material. Moreover, the pores may undergo fast collapse when the materials are exposed to an aqueous environment. Finally, depending on the loading process and interaction between loading substance and aerogel surface, active substances may be loaded in an amorphous state in form of a thin layer into the matrix (García-González et al. 2011). The given points may result in a poor kinetic control and contribute to a burst release of substances from the inner part, which is mainly controlled by the collapse of the 3D-structure instead of desired slower diffusion processes (Ulker and Erkey 2014).
One possibility to address these issues is a hydrophobic post-modification of aerogel inner surfaces, which prevents or considerably slows down the penetration of water into the pores on the one hand, but changes the original surface reactivity on the other hand. Application of a protective coating to the aerogel outer surfaces is an alternative approach, which leaves the inner part unmodified and provides a physical barrier as a protection of the inner porous structure and encapsulants. In addition, external coatings provide the possibility to achieve enhanced control over the release profile by tailoring the coating properties, like layer thickness, pH- and temperature sensitivity. Therefore, the possibility to coat cellulose aerogel particles with protective polymer layers was investigated in this work.
Application of homogeneous coating to the surface of particles is usually carried out in spray coating processes, where moving particles are brought in contact with dispersed coating solutions. Spray coating is a complex process involving many simultaneous steps and process parameters to be optimized (Jones 1985). Specifically, the light weight and low density of aerogel particles have to be considered, since they result in a non-classical fluidization behavior, which may not be described adequately by classical Geldart-classification (Akgün and Erkey 2019). The open porous nature of aerogels may allow significant penetration of the solvent into the aerogel core, resulting in changes or destruction of the porous network, due to the high capillary forces, which occur during subsequent evaporative drying. To prevent this, solidification of the polymer layer has to be faster than penetration of the solution into the pores (Alnaief et al. 2012).
Spouted bed systems are promising setups in this regard since they enable extreme fast heat and mass transfer between gas and particle phases. Aerogel particle fluidization dynamics and spray coating in spouted bed systems have already been described in work, which focused on simulation and mechanical aspects of the process, determining the important process parameters to keep particle macroscopic shape intact during processing (Antonyuk et al. 2012, 2013). Besides particle intactness, two additional requirements can be formulated for spray coating of aerogels in spouted bed systems: (1) The coating materials should be applied in a homogenous manner and defined layer thickness. (2) The inner pore structure has to be preserved to keep the aerogel functionality intact. Especially the second point is not easy to quantify via characterization methods. The shrinkage behavior of silica and starch/alginate hybrid aerogels during coating processes with an aqueous suspension was investigated by Antonyuk et al. (2015) and Alnaief et al. (2012). They also several measures for the preservation of the porous structure from shrinkage were suggested. Since the direct coating with aqueous Eudragit® solution led to pore collapse, a double layer approach was necessary, wherein the particles were firstly coated with a melt, before being exposed to water born coating material (Alnaief et al. 2012). In this regard, measurements of specific surface area can provide a sensitive assessment of aerogel quality under varying operating conditions (Subrahmanyam et al. 2015). Goslinska et al. (2019) found a direct correlation between the decrease of specific surface area and amount of applied coating material, showing the intactness of the pores and quality of the coating layer. In the same work, ethanolic shellac solutions were used to coat micrometer-sized whey protein aerogel particles with an initial surface area of 207 m2 g−1, firstly creating coated, fully edible aerogels with intact porosity.
Shellac is a natural resin and is used for instance in commercially available modified atmosphere packaging systems (Sandhya 2010). The use of edible shellac coatings as barrier system has been documented since the beginning of the last century, e.g. for pharmaceuticals, confections, fruits and vegetables. A combination of hydrophobicity and biodegradability makes shellac a promising renewable material in formulations with cellulose. In addition, cellulose/shellac systems fulfill the regulations of American food and drug administration legislations on components for food contact materials (Hult et al. 2010).
In this work we aim to investigate the possibility to coat cellulose aerogels with shellac to protect the cellulose matrix from moisture and to enable an (edible) product suitable for long term release of active substances in aqueous surrounding.
Our strategy towards shellac coated cellulose aerogel particles involves the following steps (Fig. 1): (1) Synthesis of highly spherical cellulose aerogel particles with different particle sizes, densities and narrow size distributions. Sphericity of particles is an important factor with respect to the target application, since it brings benefits in terms of flowability, homogeneous heat- and mass transfer as well as for controlled release of encapsulants (Lee et al. 2013). (2) Spray coating of cellulose aerogel particles with ethanolic shellac solutions in a miniaturized spouted bed setup under variation of process parameters like shellac content in coating solution and the ratio of a spray solution to the bed mass in order to determine successful process conditions. (3) Characterization of coating properties, coating-pore interaction and aerogel microstructure. (4) Quantification of drug delivery properties by determination of release kinetics from particles loaded in supercritical carbon dioxide.
Materials: Different coagulation bath solutions consisting of diluted (33 wt.%) aqueous sulfuric acid (H2SO4, Merck Millipore, Emsure®), and a mixture of ethyl acetate (EtAc Carl Roth GmbH & Co. KG), trichloromethane (CHCl3, Carl Roth GmbH & Co. KG) and acetic acid (CH3COOH, Carl Roth GmbH & Co. KG) were used to induce the coagulation of the cellulose solutions. EtAc and CHCl3 ratios were kept constant at equal mass ratios, CH3COOH ratio was 10 wt.% regarding the overall mass.
Preparation of cellulose aerogel particles:
A quantity of 10 or 30 g microcrystalline cellulose type II (JRS Pharma GmbH & Co. KG, Vivapure®, 101) was dispersed in 200 g demineralized water and left for swelling at 5 °C for at least 30 min.
Article information: Schroeter, B., Yonkova, V.P., Goslinska, M. et al. Spray coating of cellulose aerogel particles in a miniaturized spouted bed. Cellulose (2021). https://doi.org/10.1007/s10570-021-04032-0
Read more on Shellac as a pharmaceutical excipient here:


