Tabletability of Roll Compacted Binary Mixtures of Dicalciumphosphate with Silicified and Non-Silicified MCC
JRS Pharma presented three posters at the 2024 PBP World Meeting in Vienna. This is the second poster:
Introduction
Roll compaction/dry granulation (RCDG) is nowadays not only applied for water or heat sensitive active pharmaceutical ingredients, but has become a standard technique in pharmaceutical manufacturing . Compared to wet granulation, though, dry granules can comprise a high amount of fines and the hardness of tablets based on roller compacted granules is often reduced . Aim of the present study was, to investigate the effect of raw material choice and process setting on the quality of the granules and resulting tablets.
Material and Methods
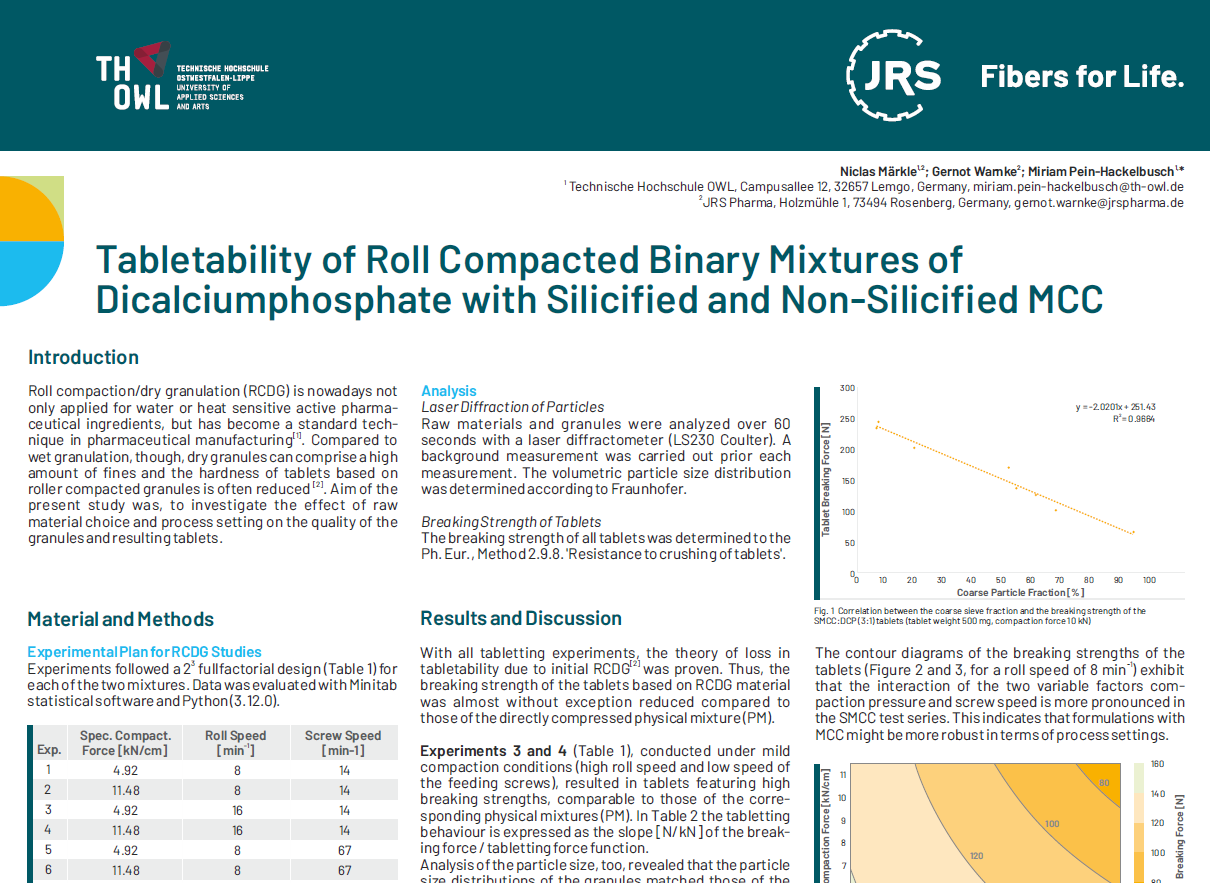
Experimental Plan for RCDG Studies
Experiments followed a fullfactorial design (Table 1) for each of the two mixtures. Data was evaluated with Minitab statistical software and Python (3.12.0).
Materials
Three materials were used for RCDG studies: microcrystalline cellulose (MCC, VIVAPUR® 101, JRS PHARMA, and silicified microcrystalline cellulose (SMCC, PROSOLV® SMCC 50, JRS PHARMA) as predominantely plastic deforming materials, and dicalcium phosphate (DCP, EMCOMPRESS® Anhydrous Powder, JRS PHARMA, as a brittle material. For the two test series, MCC:DCP (Exp.1-8a) and SMCC:DCP (Exp.1-8b) were mixed in a ratio of (3:1) in a V- mixer (JRS) for 20 min at 11 rpm.
Compaction and Tabletting
RCDG was performed with a compactor (Walzenpresse WP 50 N/75, Alexanderwerk), equipped with press rolls (Cavex CHUA 99, Flender) and a feeding screw (H4V41, Heynau Gears Production Service). The hopper agitator was constantly kept at level 5. The slugs were separated from the uncompacted fine grain before being granulated over an 800 μm sieve by a rotating mill. The compaction took place with a variable gap using a 7.5 cm long, axially profiled roller.
Tabletting of flat face tablets (13 mm diameter) was performed with an instrumented tablet press (Pressima, IMA KILIAN). Sodium stearyl fumarate (PRUV®, JRS PHARMA) was added as lubricant (1 %) to the granules (resulting from Exp.1-8a and Exp.1-8b) and mixed for 3 minutes in a cube mixer (AR 403, ERWEKA) at 24 rpm. Granules were tableted with different compaction forces in the range between 2 and 10 kN. For comparison, materials were also directly compressed as physical mixtures (MCC:DCP=PMa, SMCC:DCP=PMb), applying the same compaction forces.
Analysis
Laser Diffraction of Particles
Raw materials and granules were analyzed over 60 seconds with a laser diffractometer (LS230 Coulter). A background measurement was carried out prior each measurement. The volumetric particle size distribution was determined according to Fraunhofer.
Breaking Strength of Tablets
The breaking strength of all tablets was determined to the Ph. Eur., Method 2.9.8. ‘Resistance to crushing of tablets’.
Results and Discussion
With all tabletting experiments, the theory of loss in tabletability due to initial RCDG was proven. Thus, the breaking strength of the tablets based on RCDG material was almost without exception reduced compared to those of the directly compressed physical mixture (PM).
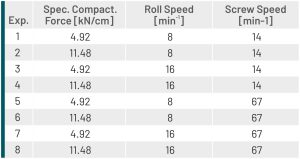
Experiments 3 and 4 (Table 1), conducted under mild compaction conditions (high roll speed and low speed of the feeding screws), resulted in tablets featuring high breaking strengths, comparable to those of the corresponding physical mixtures (PM). In Table 2 the tabletting behaviour is expressed as the slope [N/kN] of the breaking force / tabletting force function. Analysis of the particle size, too, revealed that the particle size distributions of the granules matched those of the PM (Table 2).
Experiments 5 and 6, by contrast, performed with more intense compaction (high level setting of the screw speed) resulted in tablets with lower breaking strength and bigger coarse sieve fractions.
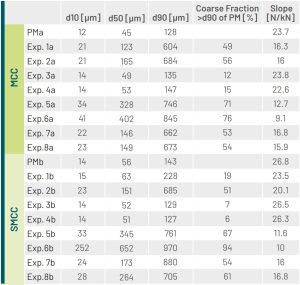
Our findings confirmed the general rule that those slugs with a higher tendency to break down into primary particles exhibit a higher tabletabilty . Furthermore, we identified an inverse correlation between the breaking strength and the coarse sieve fraction (Figure1).
The contour diagrams of the breaking strengths of the tablets (Figure 2 and 3, for a roll speed of 8 min ) exhibit that the interaction of the two variable factors compaction pressure and screw speed is more pronounced in the SMCC test series.This indicates that formulations with MCC might be more robust in terms of process settings.
Conclusion
Tabletability of granules produced with low level of screw speed and high level of roll speed was higher than the tabletability of those produced with faster screw speed and slower roll speed. The level of the specific compaction force was, however, of minor importance in the investigated setup. The general tabletability of SMCC is significantly increased compared to MCC, however, the latter is more robust in terms of process settings, especially at lower compaction forces.
Download the full poster on “Tabletability of Roll Compacted Binary Mixtures of Dicalciumphosphate with Silicified and Non-Silicified MCC” here
(click on the image to see them enlarged)
Source: JRS Pharma, PBP World Meeting, poster “Tabletability of Roll Compacted Binary Mixtures of Dicalciumphosphate with Silicified and Non-Silicified MCC“, Niclas Märkle ; Gernot Warnke ; Miriam Pein-Hackelbusch, 1 Technische Hochschule OWL, Campusallee 12, 32657 Lemgo, Germany, [email protected], 2 JRS Pharma, Holzmühle 1, 73494 Rosenberg, Germany, [email protected]
See the first JRS PBP World Meeting 2024 poster here
Do you need more information or a sample of JRS Pharma excipients?


![Fig. 2 Contourplot of the breaking force [N] of tablets (relating to a tableting compaction -1 force of 10 kN). RCDG material of MCC:DCP was produced at a constant roll speed (8 min ), varying specific compaction forces and screw speeds](https://www.pharmaexcipients.com/wp-content/uploads/2024/04/Fig.-2-Contourplot-of-the-breaking-force-N-of-tablets-relating-to-a-tableting-compaction-1024x499.jpg)
![Fig. 3 Contourplot of the breaking force [N] of tablets (relating to a tableting compaction force of 10 kN). RCDG material of SMCC:DCP was produced at a constant roll speed (8 min ), varying specific compaction forces and screw speeds](https://www.pharmaexcipients.com/wp-content/uploads/2024/04/Fig.-3-Contourplot-of-the-breaking-force-N-of-tablets-relating-to-a-tableting-compaction-1024x499.jpg)
