Tableting behavior of freeze and spray-dried excipients in pharmaceutical formulations
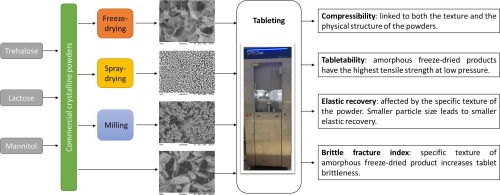
Most of biopharmaceuticals, in their liquid form, are prone to instabilities during storage. In order to improve their stability, lyophilization is the most commonly used drying technique in the pharmaceutical industry. In addition, certain applications of biopharmaceutical products can be considered by oral administration and tablets are the most frequent solid pharmaceutical dosage form used for oral route. Thus, the tableting properties of freeze-dried products used as cryo and lyoprotectant could be a key element for future pharmaceutical developments and applications. In this study, we investigated the properties that might play a particular role in the specific compaction behavior of freeze-dried excipients. The tableting properties of freeze-dried trehalose, lactose and mannitol were investigated and compared to other forms of these excipients (spray-dried, commercial crystalline and commercial crystalline milled powders). The obtained results showed a specific behavior in terms of compressibility, tabletability and brittleness for the amorphous powders obtained after freeze-drying. The comparison with the other powders showed that this specific tableting behavior is linked to both the specific texture and the physical state (amorphization) of these freeze-dried powders.
Read more here
Materials
Three excipients were used for freeze-drying, spray-drying, milling and for direct compression. Trehalose dihydrate (Biohale) and lactose (Supertab 30 Gr) were obtained from DFE Pharma (Goch, Germany), mannitol from Cooper (Melun, France) and Magnesium stearate (Ligamed MF-2-V) from Peter Greven (Bad Münstereifel, Nordrhein-Westfalen, Germany).
Charbel Madi, Hassana Hsein, Virginie Busignies, Pierre Tchoreloff, Vincent Mazel, Tableting behavior of freeze and spray-dried excipients in pharmaceutical formulations, International Journal of Pharmaceutics, Volume 656, 2024, 124059, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124059.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


