Topical co-delivery of tacrolimus and calcipotriol loaded nanostructured lipid carrier: a potential and synergistic approach in the management of psoriasis
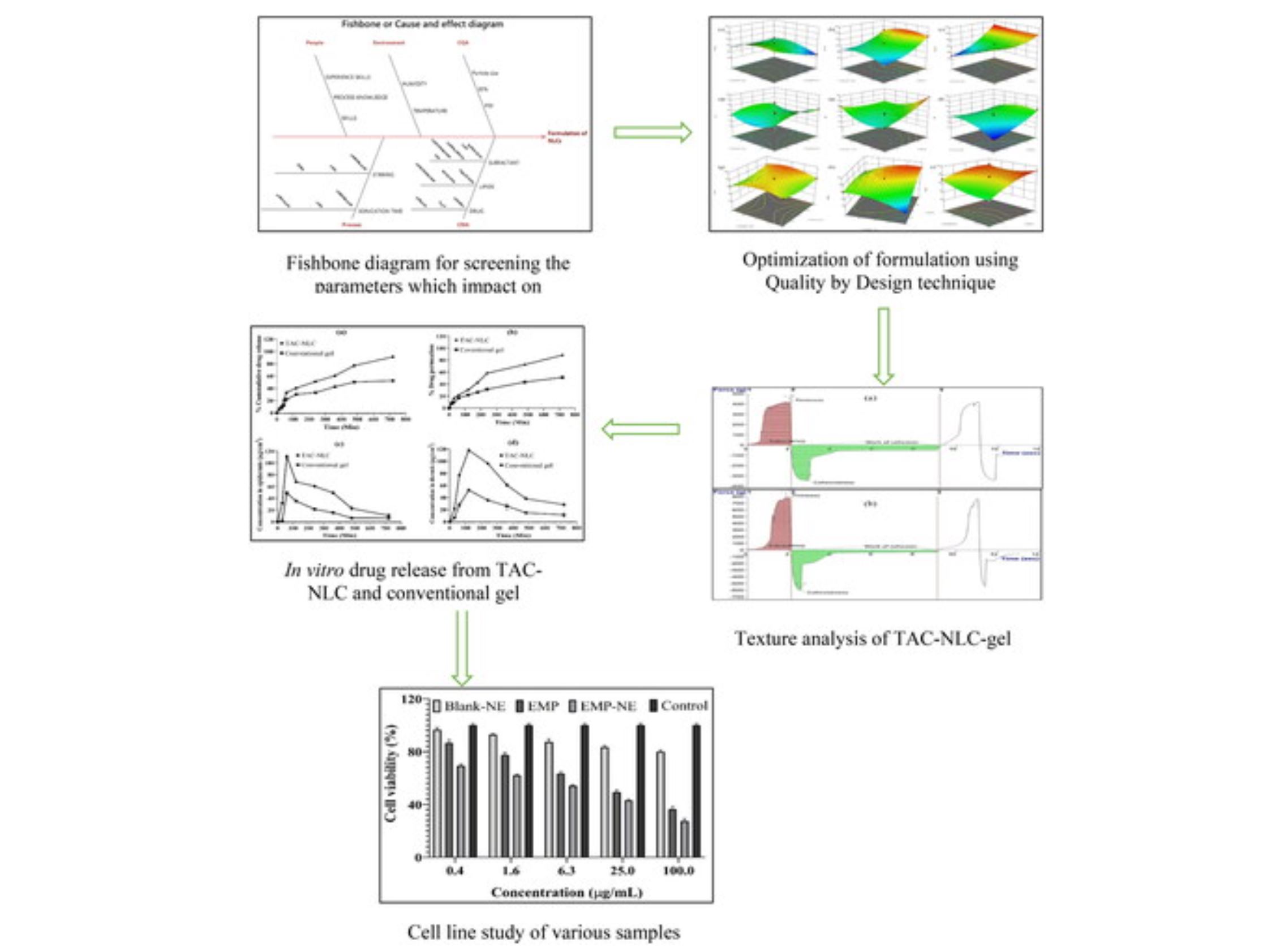
The focus of this study was to develop a Vitamin-D3 (Calcipotriol)-based Tacrolimus (TAC)-loaded nanostructured lipid carrier (NLC) for topical application to treat psoriasis. The sonication technique followed by melt emulsification was used to formulate TAC-NLC, in which Precirol ATO-5, Calcipotriol, and Solutol HS-15 were used as excipients. Based on the analysis of particle size, PDI, and entrapment efficiency of various TAC-NLCs using Box-Behnken Design, an optimized NLC was selected. The selected nanocarrier was further transformed into the gel and then characterized for in vitro release, in vitro and ex vivo permeation, and dermatokinetic, including in vitro characterization studies.
The optimized TAC-NLC had a particle size of 231 ± 1.52 nm with a narrow PDI of 0.40 ± 0.001 along with high entrapment efficiency (84.04 ± 1.36%) and drug loading (12.61 ± 0.20%)”. The in vitro release showed sustained release throughout 12 h. Moreover, dissimilarity (f1:52) and the similarity factor (f2:37) showed a lack of any similarity in the evaluation of drug release from TAC-NLC formulation and Conventional formulation. The time requisite to release 50% (T50), 30% (T30), and 20% (T20) of the drug is also calculated. The flux and permeability coefficients were found to be 11.59 µg/cm2/h and 0.0386 cm/h.
A dermatokinetic study of TAC-NLC indicated a remarkable rise in AUC0-12h and CSkin max compared to conventional formulation (p < 0.005). Further, the vitro studies indicated higher cell viability (85%-80%) on melanoma cells than aqueous suspension of the drug (60–70%). The study indicated that the topical application of TAC-NLC nanogel was found potentially useful for the management of psoriasis.
Read more here
Topical co-delivery of tacrolimus and calcipotriol loaded nanostructured lipid carrier: a potential and synergistic approach in the management of psoriasis, Received 17 Nov 2023, Accepted 24 Feb 2024, Published online: 11 Mar 2024, https://doi.org/10.1080/01932691.2024.2325395
Read also our introduction article on Topical Excipients here:


