Taste Masked Artesunate/Amodiaquine Micropellets in the Fight Against Malaria
This article shows the content of a poster which was presented at the 𝗘𝗨𝗣𝗳𝗜 𝗰𝗼𝗻𝗳𝗲𝗿𝗲𝗻𝗰𝗲 𝗶𝗻 𝗚𝗹𝗮𝘀𝗴𝗼𝘄 2023
Malaria & Treatment Challenges
Children aged under 5 years are the worst affected by malaria, causing about 10 % of all children’s deaths in regions where malaria is endemic. Fixed dose combination (FDC) of Artesunate (AS) and Amodiaquine (AQ), are recommended by the WHO for malaria treatment. Currently AS/AQ is only available as bilayer tablets which are crushed for paediatric administration causing noncompliance due to the bitter taste of AQ. The aim of this work is to develop a FDC of AS/AQ taste masked micropellets (diameter <250 µm) with improved palatability and mouthfeel for oral administration in young children. The manufacturing of effectively taste masked micropellets is facilitated using the MicroCoat™ technology.
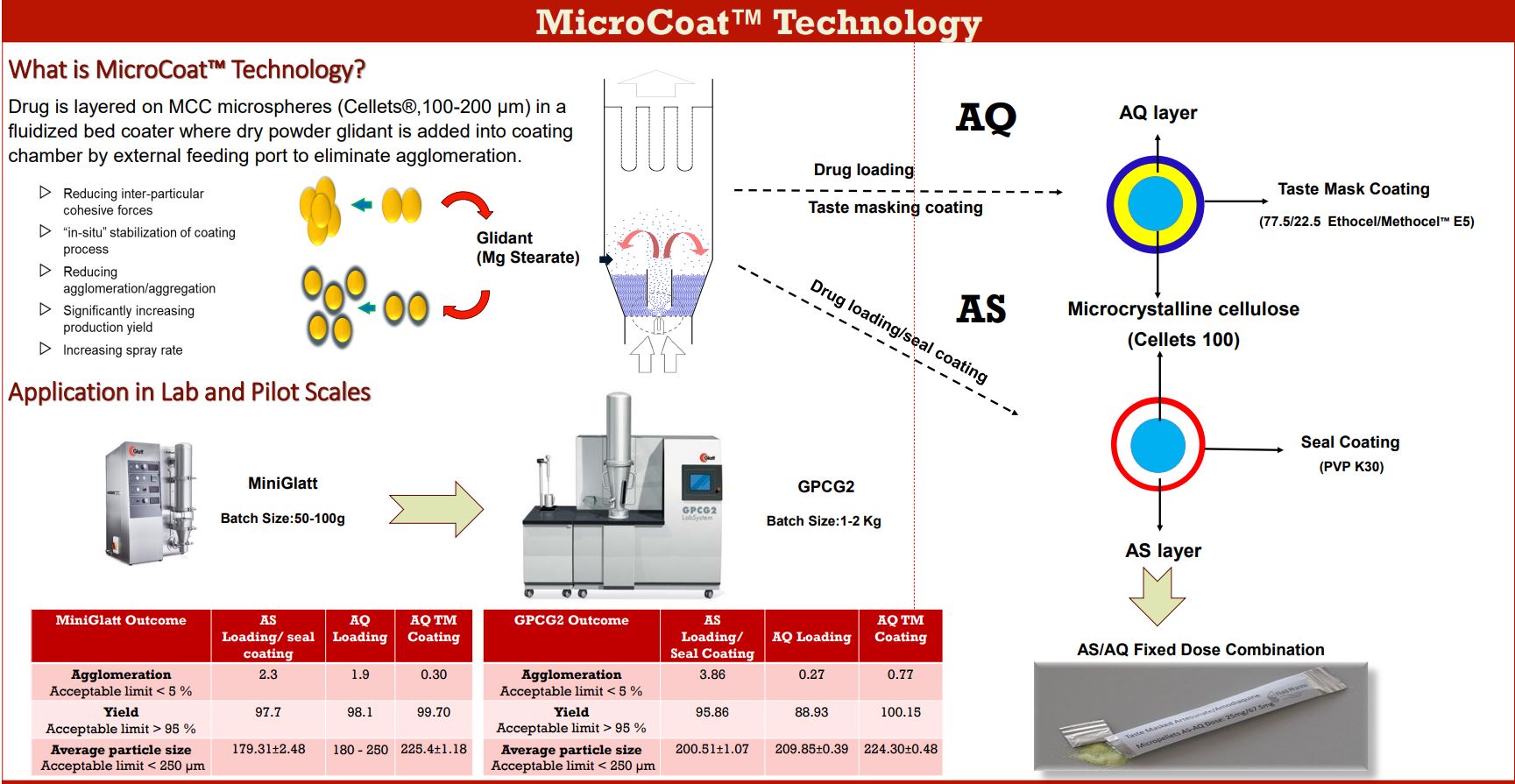
MicroCoat™ Technology
What is MicroCoat™ Technology?
Drug is layered on MCC microspheres (Cellets®,100-200 µm) in a fluidized bed coater where dry powder glidant is added into coating chamber by external feeding port to eliminate agglomeration.
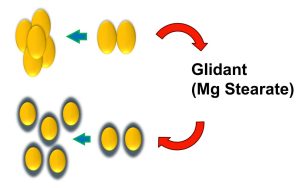
- Reducing inter-particular cohesive forces
- “in-situ” stabilization of coating process
- Reducing agglomeration/aggregation
- Significantly increasing production yield
- Increasing spray rate
Application in Lab and Pilot Scales
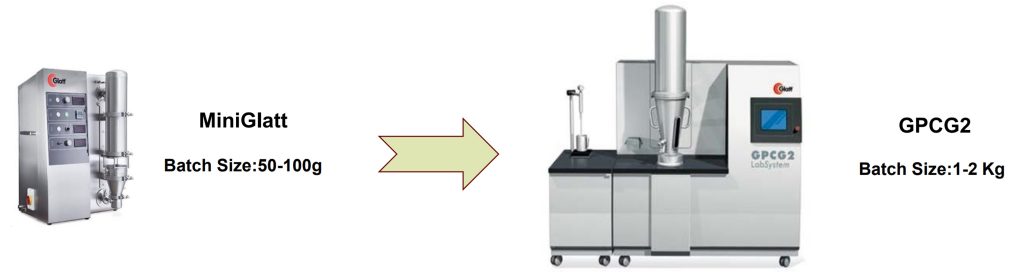
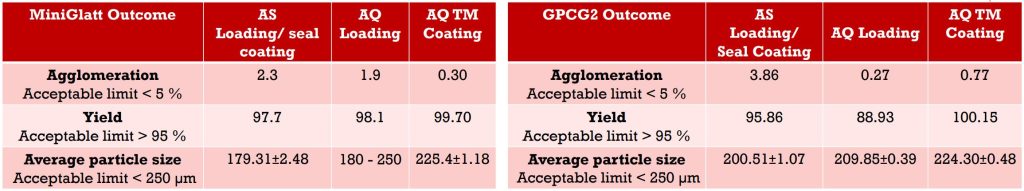
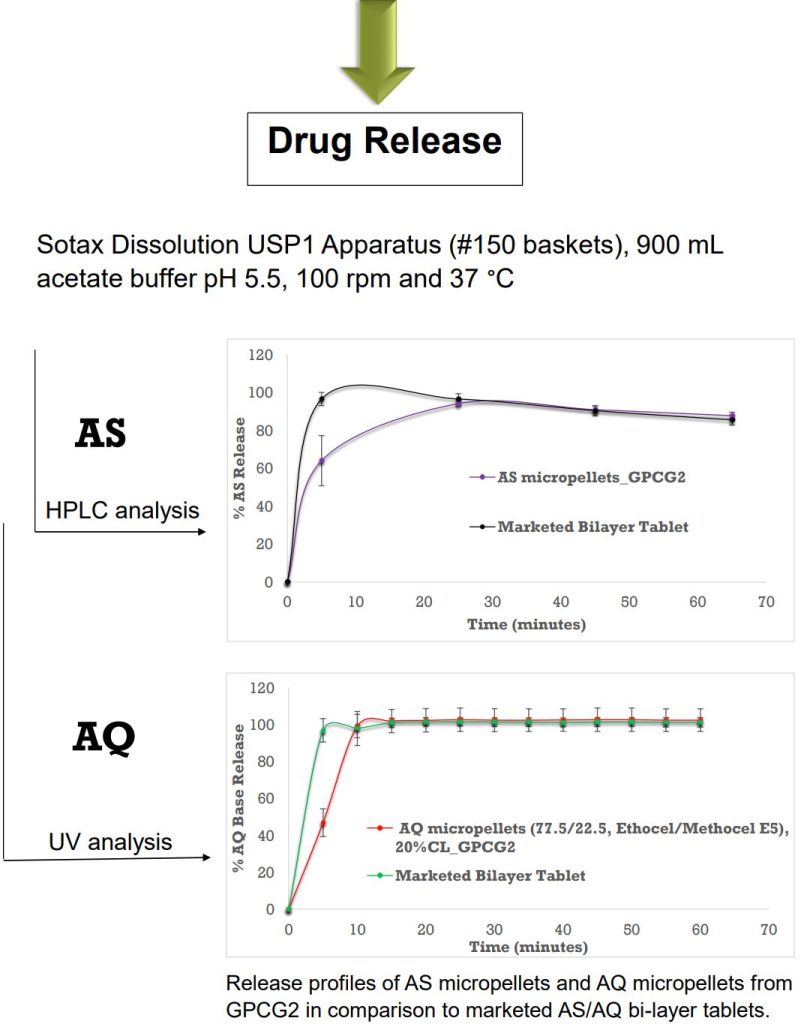
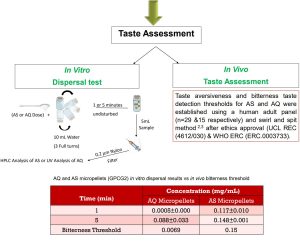
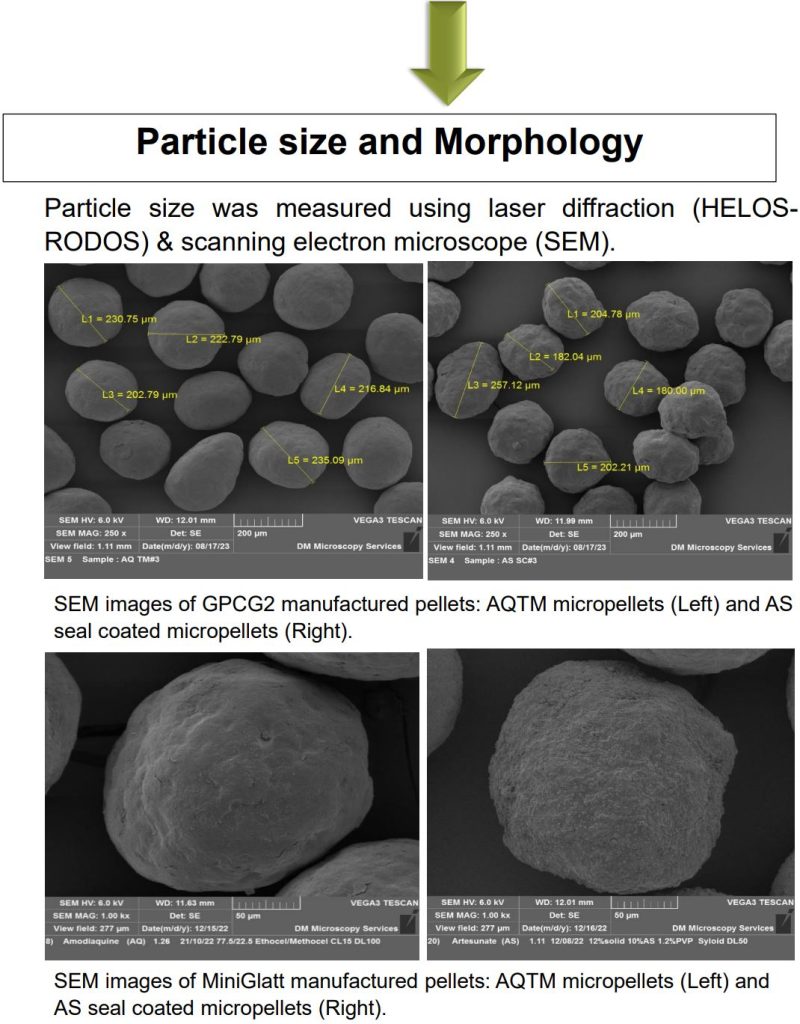
Final Product Evaluation
Excipient Safety Evaluation
Total excipients intake was calculated at two dose strengths: 25 mg/67.5 mg and 50 mg/135 mg (AS/AQ) for use in children 4.5 kg to <18 kg, corresponding to (2 months-5 years). Excipients safety profiles were evaluated against the “Generally Regarded as Safe” (GRAS), “Safety and Toxicity of Excipients for Paediatrics” (STEP) and “FDA Inactive Ingredient” databases. No excipient safety concerns were raised following the evaluation process to be used in the target population.
Conclusions
An effective taste masked coating was successfully applied on drug loaded micropellets using MicroCoat™ technology on both laboratory and pilot scale giving an acceptable paediatric ASAQ FDC product which can improve adherence of anti-malarial treatments in children under 5 years. The final formulation will be further assessed by acceptability and biorelevant dissolution studies.
See the full poster on “Taste Masked Artesunate/Amodiaquine Micropellets in the Fight Against Malaria” here
(click the picture to enlarge the poster)
Source: Fluid Pharma, Unitaid,
Authors: Alan Reader, Dina Shokry, Annette Grave, Janosch Kilnger, Maurice Wilde,Dan Baker, Sangeetha Marri, Catherine Tuleu, Sejal Ranmal, Daniel Schaufelberger, Harun Sinha, Richard Lasky, Sandra Klein, Frank Karkossa, Kavil Patel, Stephanie De Sa, Marques Basset & Fang Liu
Poster “Taste Masked Artesunate/Amodiaquine Micropellets in the Fight Against Malaria” 𝗳𝗿𝗼𝗺 𝘁𝗵𝗲 𝗘𝗨𝗣𝗳𝗜 𝗰𝗼𝗻𝗳𝗲𝗿𝗲𝗻𝗰𝗲 2023 𝗶𝗻 𝗚𝗹𝗮𝘀𝗴𝗼𝘄