Nanostructured Lipid Carriers for Improved Delivery of Therapeutics via the Oral Route
Abstract
Drug delivery via the oral route has always been challenging for poorly soluble drugs. Acid-induced hydrolysis, enzymatic degradation, and poor mucosal absorbency remain the primary hiccups for effective oral delivery of medications. With the advent of nanotechnology, nanostructured lipid carriers (NLCs) have emerged as a promising delivery carrier that can circumvent gastrointestinal tract (GIT) barriers hindering the solubility and bioavailability of such drugs. These NLCs can efficiently transport drug moieties across intestinal membranes shielding medications from intestinal pH and enzymatic degradation. Because they are composed of lipidic materials, they can be easily absorbed or taken up by various pathways such as transcellular absorption, paracellular transport, and M-cell uptake. Such mechanisms not only improve the absorption and solubility of drugs but also augment bioavailability and residence time and may bypass first-pass metabolism. This review explores the diverse applications of nanostructured lipid carriers (NLCs) in oral drug delivery for various medical conditions, shedding light on their current regulatory status, including FDA-approved options and those in pre/clinical stages. The review also features patented NLC formulations. It provides valuable insights into how NLCs can be harnessed for effective oral drug delivery and outlines recent advancements in optimizing their performance to tackle gastrointestinal barriers, thus opening new possibilities for NLCs in future pharmaceutical applications.
Introduction
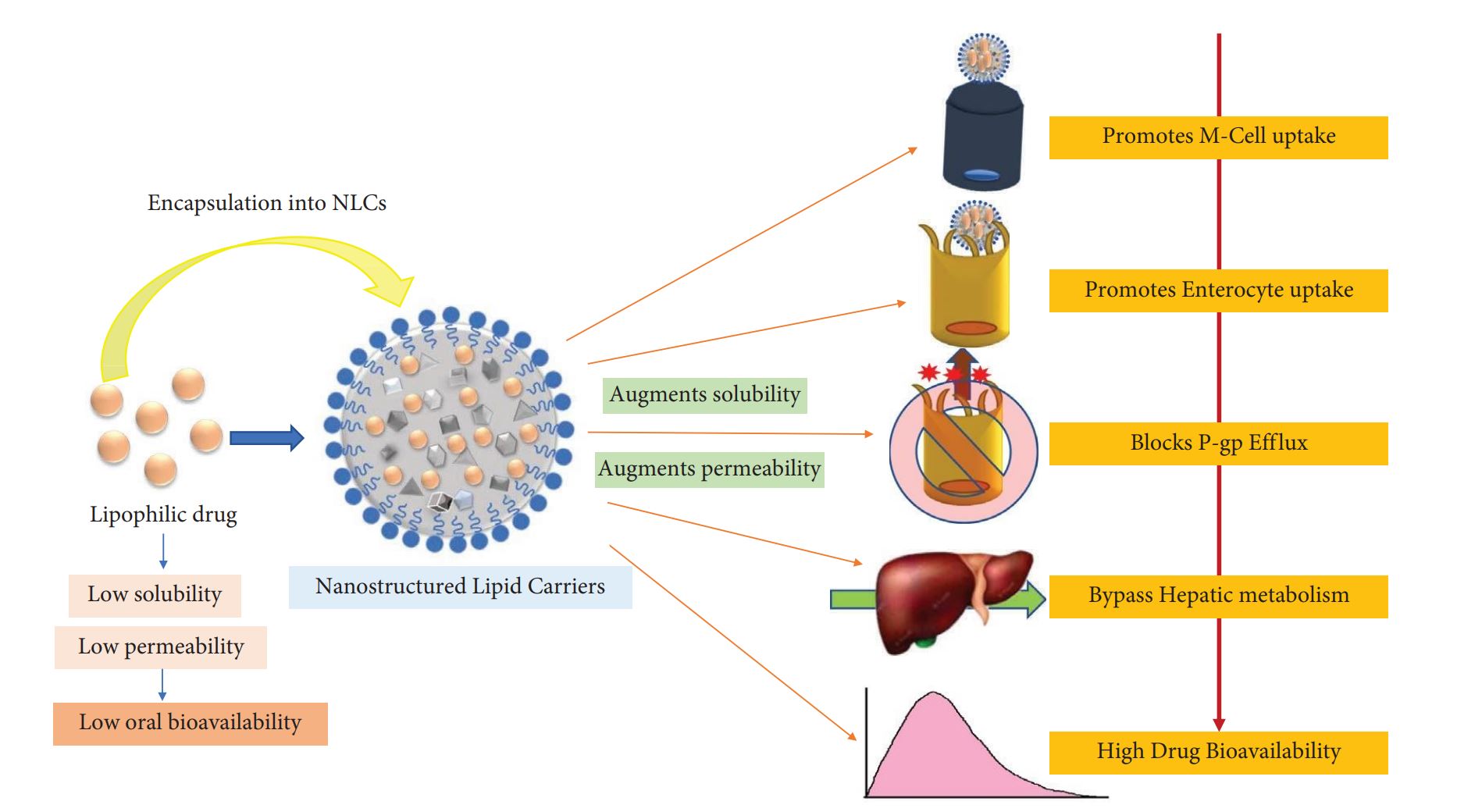
Delivery of medications via the oral route is considered the ideal way to achieve therapeutic and prophylactic effects against many ailments in treating both acute and chronic conditions [1]. In addition, oral delivery possesses several benefits such as noninvasiveness, ease of administration, patient compliance, and economical and ease of large-scale manufacturing. Drugs that are capable of sustaining stability in the stomach acidic environment and do not pose gastrointestinal (GI) irritation and toxicity challenges are preferred for oral delivery [2, 3]. Although most drugs are lipophilic, poor absorption leading to low bioavailability is a major concern regarding the formulation of a successful oral dosage form.
Furthermore, poor permeability across the GI membrane [2, 3], intrinsic dissolution rate (mass of the drug dissolved per time unit and area), where dissolution is the rate-limiting step in the absorption of hydrophobic drugs (especially drugs of BCS class II and IV) [4], acidic environment [5], first-pass metabolism, intraenterocyte metabolism, and enzymatic degradation [6] limit the absorption of the therapeutic agent. Also, drug eviction from the drug transporter (P-glycoprotein: P-gp) and interaction with the food present in the GIT leads to variable absorption of the drug, finally reaching systemic circulation [6]. In addition, the age, gender, and pathological condition of patients affect the absorption of the drug.
Several approaches have been explored to augment the solubility of BCS class II and IV drugs. One such method is the transformation of the drug into a solubilized state, enabling the absorption profile of the drug to be close to that of the BCS class I drug [7]. Another approach is the use of nanotechnological approaches such as polymeric nanoparticles, microspheres, lipid-polymer hybrid nanoparticles (LPHNPs) [8], lipid-based nanoparticles [9] such as liposomes, niosomes, and solid lipid nanoparticles (SLNs) [10], and nanostructured lipid carriers (NLCs) for improving the solubility of drugs [11, 12].
NLCs are colloidal structures comprising an amalgamation of solid and liquid lipids that constitute an amorphous lipid matrix fenced by a solid lipid coat. A combination of solid and liquid lipids provides structural integrity to NLCs where less organized structures have been created that accord a steadier enclosure of the drug in the lipid matrix lending long-term shelf life to the formulation [13]. In addition, NLCs can incorporate both hydrophilic and hydrophobic therapeutics [14].
NLCs present several advantages such as easy manufacturing, low toxicity, physical stability, custom-tailored release, high drug entrapment, no drug leaching during storage, and improvement of drug’s solubility and stability, which are some excellent features that grant them an upper hand over other drug delivery systems. NLCs, by their biocompatible nature, can be administered via the oral, parenteral, topical, rectal, and pulmonary routes [15–17].
Owing to the several advantages of NLCs, this review focuses on the events that occurred late and recently in the successful oral delivery of poorly soluble medications using NLCs. In this article, we present an understanding of the mechanism of drug protection in terms of in vitro-in vivo capacity, preparation methods of NLCs, and applicability in conveying a variety of medications, proteins and peptides, bioactive compounds, etc., across biomembranes. In addition, insights on the clinical trials and patents granted on the potential implications of NLCs in the delivery of therapeutics are provided.
Lipid-based nanoparticles have been utilized in the delivery of poorly soluble drugs of late with solid lipid nanoparticles being the earlier drug delivery system that has shown great potential in the delivery of medications across the GIT. The use of biodegradable natural lipids and surfactants and toxic solvent-free methodologies in their preparation enable lipid-based nanoparticles to be the foremost selection for the delivery of poorly soluble drugs. The natural lipids used can withstand the degradation factors both in vitro and in vivo rendering them the first choice for nanoparticulate drug delivery systems that deliver medications across biological membranes [14]. According to the data available on Google Scholar, more than twenty-four thousand pieces of literature are available on lipid nanoparticles for oral delivery from 2010 to date, indicating their extensive use in the transport of biological molecules.
Lipid nanoparticles are now being considered as a substitutional food supplement post-COVID-19 [18] and are now the fast-growing sector worldwide with a compound annual growth rate (CAGR) of 13.6% between 2022 and 2029, which is expected to rise from $777.4 million in 2022 to $1,895.1 million by 2029, due to increasing health alarms [19]. People are interested in using these lipid nanoparticles because of the presence of omega-3 and omega-6 as main components. Therefore, lipid-based nanoparticles have huge potential for use in pharmaceutical, healthcare, dietary supplements, functional food/beverages, and cosmetics/personal care sectors. SLNs share the market with a market share of 45% [20].
Table 1: List of solid/liquid lipids, surfactants, and surface modifers commonly used in the fabrication of NLCs.
| Solid lipids | Melting point (°C) |
|---|---|
| Stearic acid | 67-69 |
| Behenic acid | 80 |
| Palmitic acid | 83 |
| Lauric acid | 43 |
| Carnauba wax | 78-88 |
| Goat wax | 40-50 |
| Beeswax | 62-64 |
| Teobroma oil | 35-37 |
| Tristearin/glyceryl tristearate | 70-74 |
| Cetyl palmitate | 54 |
| Glyceryl monostearate | 54-64 |
| Imwitor 372P, 491, 900k, 928 | 61–77, 34 |
| Glyceryl palmitostearate | 50-60 |
| Glyceryl behenate | 65-77 |
| Trilaurin (Dynasan 112) | 43-46 |
| Trimyristin (Dynasan 114) | 55-58 |
| Tristearin (Dynasan 118) | 70-73 |
| Tripalmitin (Dynasan 116) | 61-65 |
| Tribehenate (Dynasan 122) | 81-85 |
| Hydrogenated palm oil (Dynasan P60) | 58-62 |
| Hydrogenated palm oil (Softisan 154) | 53-58 |
| Softisan 100, 138, 142, 154, 378, 601, 645, 649 | 33-58 |
| Witepsol (E, W, S, H) | 31-44 |
| Liquid lipids | Viscosity (mPas at 20–30°C) |
| Miglyol (808, 810N, 812N, 818) | 23-33 |
| Capric acid | 25-33 |
| Caprylic acid | 26-32 |
| Oleic acid | 40 |
| Fatty acid esters | 6.57 |
| Propylene glycol fatty acid esters | 6.98 |
| Mineral oil | 95-100 |
| Vitamin E | NA |
| Olive oil | 85 |
| Castor oil | 390 |
| Palm oil | 130 |
| Coconut oil | 85 |
| Soybean oil | 55 |
| Jojoba oil | 33.3 |
| Mustard oil | 117 |
| Garlic oil | 80 |
| Clove oil | 9 |
| Emulsifers/coemulsifers | HLB value |
| Polysorbate 20, 80 | 15-17 |
| Solutol HS | 15 |
| Poloxamer 188 | 29 |
| Poloxamine 908 | 31 |
| Cremophor El | 12-17 |
| Sodium cholate | 18 |
| Sodium dodecyl sulfate | 40 |
| Polyvinyl alcohol | 18 |
| Sodium oleate | 18 |
| Soy lecithin | 4 |
| Egg lecithin | 6.6 |
| Lecithin | 3-5 |
| Surface innovators | |
| Wheat germ agglutinin | |
| Hyaluronic acid | |
| Mannose | |
| β-d-galactosides | |
| Ferritin | |
| Transferrin | |
| Biotin | |
| L-arginine | |
| Oligochitosan | |
| Polyethylene glycol | |
| Folic acid |
Download the full article as PDF here Nanostructured Lipid Carriers for Improved Delivery of Therapeutics via the Oral Route
or read it here
Other excipients named in the study: Imwitor 900 K , Dynasan 116 (tripalmitin), Kolliwax GMS II (glycerol monostearate), Compritol 888 ATO (glyceryl dibehenate), and cetostearyl as the solid lipid components of NLCs. As surfactants: d-α-tocopherol polyethylene glycol succinate, vitamin E polyethylene glycol succinate or vitamin E-TPGS, poloxamer 188, Tween® 80
Hindawi, Journal of Nanotechnology, Volume 2023, Article ID 4687959, 35 pages, https://doi.org/10.1155/2023/4687959, Nanostructured Lipid Carriers for Improved Delivery of Therapeutics, Alok Kumar Mahor, Prem Prakash Singh, Rishikesh Gupta, Peeyush Bhardwaj, Priyanka Rathore, Ankita Kishore, Rohit Goyal, Neeraj Sharma, Jyoti Verma, Jessica M. Rosenholm and Kuldeep K. Bansal, Received 8 August 2023; Revised 20 September 2023; Accepted 29 September 2023; Published 6 November 20

