Design of Redispersible High-Drug-Load Amorphous Formulations: Impact of Ionic vs Nonionic Surfactants on Processing and Performance
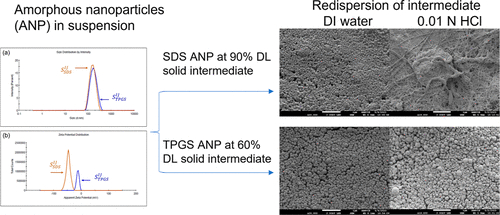
Amorphous solid dispersions (ASDs) are an enabling formulation approach used to enhance bioavailability of poorly water-soluble molecules in oral drug products. Drug-rich amorphous nanoparticles generated in situ during ASD dissolution maintain supersaturation that drives enhanced absorption. However, in situ formation of nanoparticles requires large quantities of polymers to release drugs rapidly, resulting in an ASD drug load <25%. Delivering directly engineered drug-rich amorphous nanoparticles can reduce the quantities of polymers significantly without sacrificing bioavailability. Preparation of 90% drug-load amorphous nanoparticles (ANPs) of <300 nm diameter using solvent/antisolvent nanoprecipitation, organic solvent removal, and spray drying was demonstrated previously on model compound ABT-530 with Copovidone and sodium dodecyl sulfate (anionic). In this work, nonionic surfactant d-α-tocopheryl polyethylene glycol succinate (Vitamin E TPGS, or TPGS) was used to prepare ANPs as a comparison. Characterization of ANPs by dynamic light scattering, filtrate potency assay, scanning electron microscopy, and differential scanning calorimetry revealed differences in surface properties of nanoparticles afforded by surfactants. This work demonstrates the importance of understanding the impact of the stabilizing agents on nanoparticle behavior when designing a high-drug-load amorphous formulation for poorly water-soluble compounds as well as the impact on redispersion.
Read more
Design of Redispersible High-Drug-Load Amorphous Formulations: Impact of Ionic vs Nonionic Surfactants on Processing and Performance, Mengqi Yu, Hardeep S. Oberoi, Hitesh S. Purohit, Craig A. Fowler, and Devalina Law, Molecular Pharmaceutics Article ASAP
https://doi.org/10.1021/acs.molpharmaceut.3c00684

