VITAMIN E – Direct Compression with PROSOLV 730

Aim of the study
Solid dose formulations of Vitamin E, an oily active ingredient, are typically produced as soft-gel capsules in the pharmaceutical and nutraceutical industry. The manufacturing process of these soft-gel capsules is labor-intensive and costly. The goal of this study was to create directly compressible 100 IU (67 mg) Vitamin E tablets with minimal effort.
Vitamin E

Vitamin E is a fat-soluble vitamin with potent antioxidant properties found in many foods, including poultry, eggs, fruits, and vegetables. Research shows that Vitamin E protects cells from oxidative stress, regulates immune function, maintains [1] endothelial cell integrity, and balances normal coagulation. Vitamin E supplements can help prevent coronary heart disease, support the immune system, prevent inflammation, promote eye [2] health, and lower the risk of cancer.
Formulation
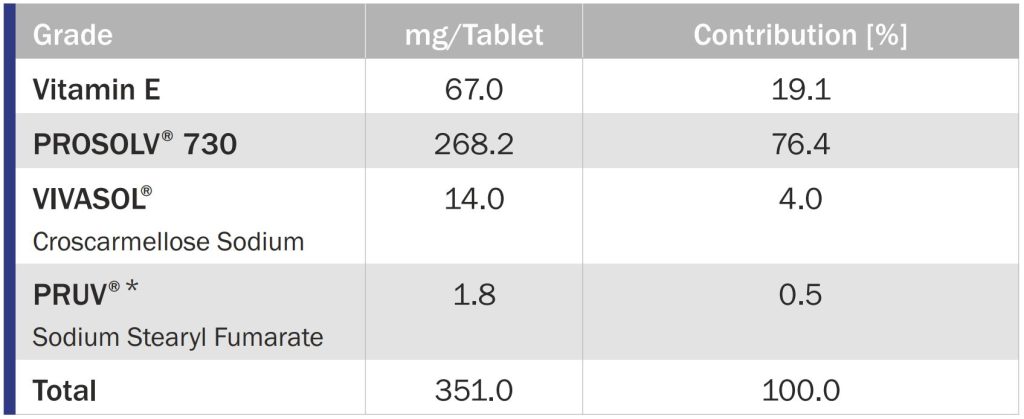
The tablet formulation consisted of Vitamin E, the high functionality adsorbant binder PROSOLV 730, VIVASOL croscarmellose sodium as disintegrant, and PRUV sodium stearyl fumarate as lubricant.
Vitamin E (DL-α-Tocopherol Acetate >98 %) was obtained from TCI America, Inc. (Figure 1).
Excipients
PROSOLV 730 is a high functionality excipient composite that was specifically designed to adsorb oily Active Pharmaceutical Ingredients (APIs), resulting in a free flowing powder, capable of being compressed into tablets or encapsulated. Combining PROSOLV 730 with liquid APIs and additional excipients, such as VIVASOL CCS as disintegrant, and PRUV SSF or LUBRI-PREZ magnesium stearate as a lubricant, conveniently enables direct compression of tablets, avoiding costly soft-gel encapsulation processes.
Procedure
Blending
Vitamin E was added to PROSOLV 730 via high shear mixing for 10 minutes. The oil-loaded PROSOLV 730 was then transferred to a low shear mixing vessel and blended with the disintegrant VIVASOL CCS for 5 minutes. PRUV SSF was sieved through a 20 mesh screen, then added to the low shear mixer, and blended for an additional 5 minutes. The blend was immediately used for direct compression tableting.
Equipment
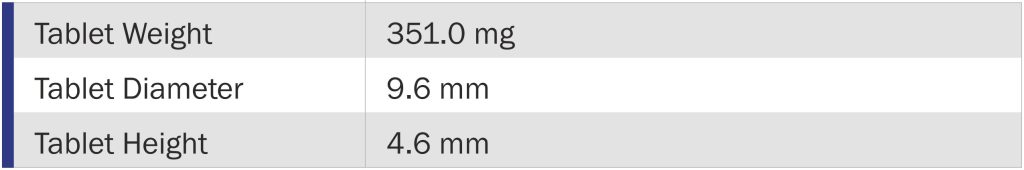
Tablet Characteristics
Conclusion
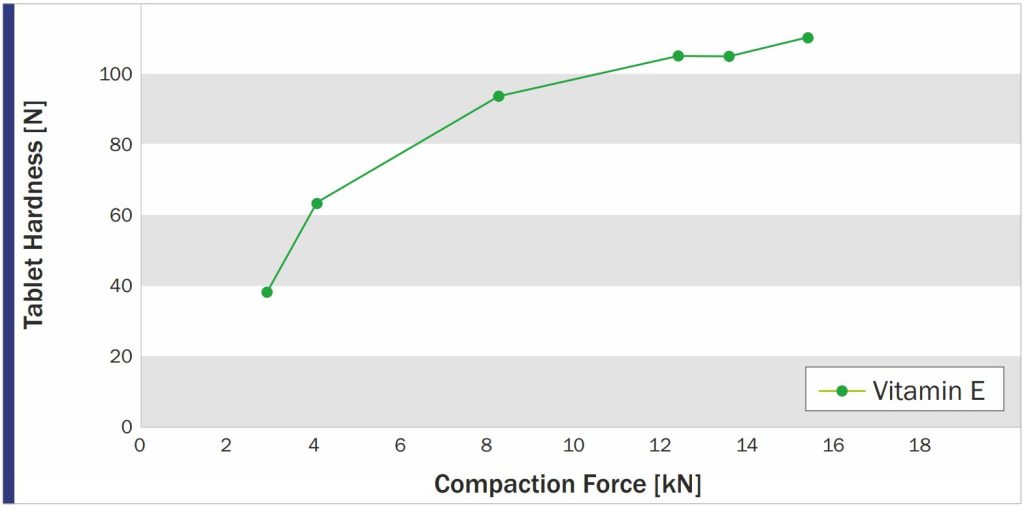
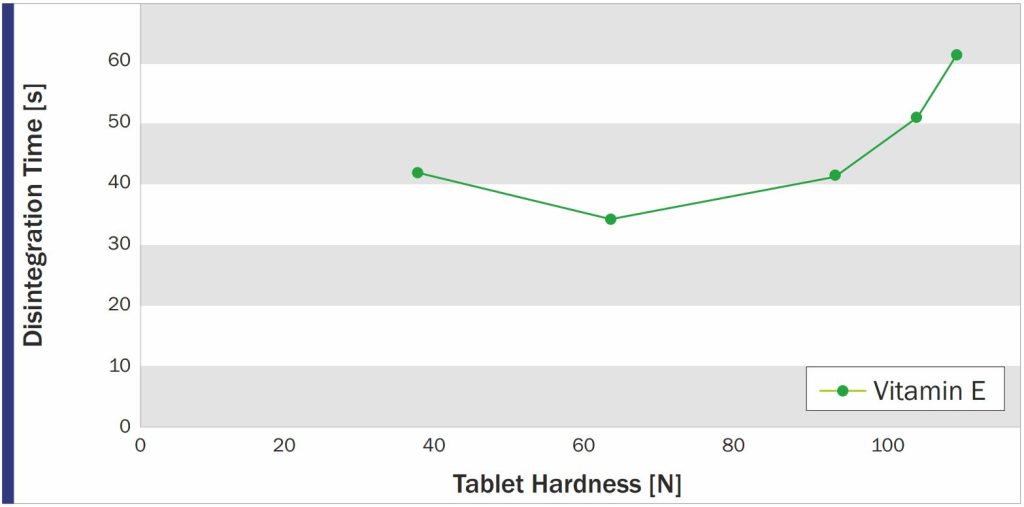
PROSOLV 730 was found to be perfectly suited for the production of Vitamin E tablets by direct compression. The tablets showed sufficient hardness – obtained by moderate compression force – and fast disintegration.
PROSOLV 730 enables simple, direct compression formulation of oily APIs, thus offering time and cost savings compared to soft-gel encapsulation.
See the full formulation study on “VITAMIN E – Direct Compression with PROSOLV 730” here
(click the picture to download the brochure)
Source: JRS Pharma technical brochure “VITAMIN E – Direct Compression with PROSOLV 730“
Do you need more information or a sample of JRS Pharma excipients?