Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery
Bacterial conjunctivitis is a worldwide problem that, if untreated, can lead to severe complications, such as visual impairment and blindness. Topical administration of ciprofloxacin is one of the most common treatments for this infection; however, topical therapeutic delivery to the eye is quite challenging. To tackle this, nanomedicine presents several advantages compared to conventional ophthalmic dosage forms. Herein, the flash nanoprecipitation technique was applied to produce zein and hyaluronic acid nanoparticles loaded with ciprofloxacin (ZeinCPX_HA NPs). ZeinCPX_HA NPs exhibited a hydrodynamic diameter of <200 nm and polydispersity index of <0.3, suitable for ocular drug delivery. In addition, the freeze-drying of the nanoparticles was achieved by using mannitol as a cryoprotectant, allowing their resuspension in water without modifying the physicochemical properties. Moreover, the biocompatibility of nanoparticles was confirmed by in vitro assays. Furthermore, a high encapsulation efficiency was achieved, and a release profile with an initial burst was followed by a prolonged release of ciprofloxacin up to 24 h. Overall, the obtained results suggest ZeinCPX_HA NPs as an alternative to the common topical dosage forms available on the market to treat conjunctivitis.
Introduction
Conjunctivitis affects many people worldwide and consists of the inflammation and swelling of the conjunctival tissue, as well as dilation of the blood vessels, ocular discharge, and discomfort. Conjunctivitis can be divided into four main groups based on the etiology: bacterial, viral, allergic, and irritant [1,2]. Bacterial conjunctivitis is the second-most common infectious conjunctivitis and is more frequent in children [3]. Further, bacterial conjunctivitis is one of the most common ophthalmic diseases in developed countries [4]. Several bacterial are etiological agents of conjunctivitis, the most common of which being Streptococcus pneumoniae, Haemophilus influenza, Moraxella catarrhalis, and Staphylococcus aureus; the last is more common in adults [5]. Ciprofloxacin (CPX) is one of the most-used antibiotics in the treatment of bacterial conjunctivitis [6], and is a broad-spectrum antibiotic that belongs to a class of antibiotics designed by fluoroquinolones [7]. Fluoroquinolones have excellent antibacterial effects against Gram-negative and many Gram-positive bacteria [8]. Nevertheless, commercial CPX eye drop solutions have an acidic pH, which causes local burning and itching [9,10]. Furthermore, the low solubility of CPX under ocular physiological conditions (pH ≈ 7) leads to a lower drug bioavailability [9].
Up to the present, topical dosage forms were elected as a less invasive administration route to the ocular mucosa. However, obstacles such as tear fluid production and the corneal barrier limit drug bioavailability. To tackle this challenge, researchers have proposed nanoparticles (NPs)-based systems for treating ocular infections, as reviewed by Liu et al. [11]. These systems can increase the retention time of the drugs on the ocular surface and protect them from enzymatic degradation, while simultaneously contributing to the decrease of the drug concentration administered to assure the therapeutic effect [12,13,14]. In addition, other authors demonstrated that by incorporating drugs into NPs, there is an enhancement of corneal permeability [11,15]. Hence, flash nanoprecipitation (FNP) is a simple and effective approach to producing NPs with high drug-encapsulation efficiency (EE) [16]. The FNP technique is based on a rapid mixing that creates high-supersaturation conditions, leading to the precipitation and encapsulation of both hydrophobic and hydrophilic drugs into polymeric NPs [16,17,18]. Several studies demonstrated that FNP allows the encapsulation of hydrophobic and hydrophilic drugs with high EE, as we have previously reported [18,19].
Herein, ZeinCPX_HA NPs were prepared using the FNP technique. Zein is a water-insoluble protein extracted from corn and is generally recognized as safe (GRAS) by the FDA [20]. In fact, zein has been widely explored in biomedical applications, namely in the field of pharmaceuticals, due to its physicochemical and biological properties [21]. Further, zein has been widely applied as a drug carrier due to its biocompatibility and amphiphilic nature which promote the self-assembly process [18,22,23,24] and the encapsulation of poorly water-soluble compounds [25]. On the other hand, hyaluronic acid (HA) is a polysaccharide selected due to its mucoadhesive character so that it will increase the pre-corneal residence of the drug [26]. Therefore, the pre-corneal clearance will be reduced by using HA, and consequently, a higher cellular interaction and ocular bioavailability will be attained [27]. Moreover, HA is a ligand for the CD44 receptor, which is present in the human cornea and conjunctiva. Under some pathological and inflammatory conditions, the CD44 receptor number increases, prompting the interaction with HA [28,29,30,31].
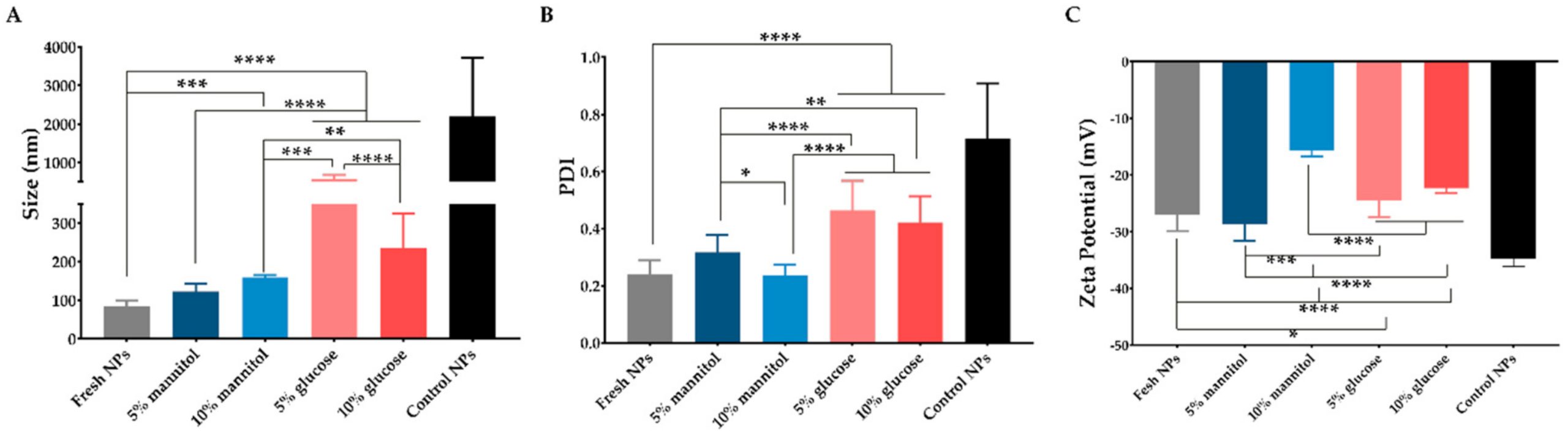
To the best of our knowledge, this is the first report of ZeinCPX_HA NPs produced by FNP. In the first instance, the polymer ratio (zein and HA) and cryoprotectants (glucose and mannitol) were optimized to obtain stable lyophilized NPs suitable for ocular drug delivery. These cryoprotectants can act as protective agents during freezing due to an increase in the surface tension of the water molecules, and can also work as cryoprotectants by preventing stress during the drying phase [32,33].
Thus, the main purpose of this study was to develop biocompatible polymeric NPs suitable for CPX ocular delivery with enhanced bioavailability and stability under long-term storage.
Download the full article as PDF here Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery
or read it here
Excipients used in this research besides other: Mannitol, Zein
Jacinto, T.A.; Oliveira, B.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Ciprofloxacin-Loaded Zein/Hyaluronic Acid Nanoparticles for Ocular Mucosa Delivery. Pharmaceutics 2022, 14, 1557. https://doi.org/10.3390/pharmaceutics14081557

