3D-printing of solid lipid tablets from emulsion gels
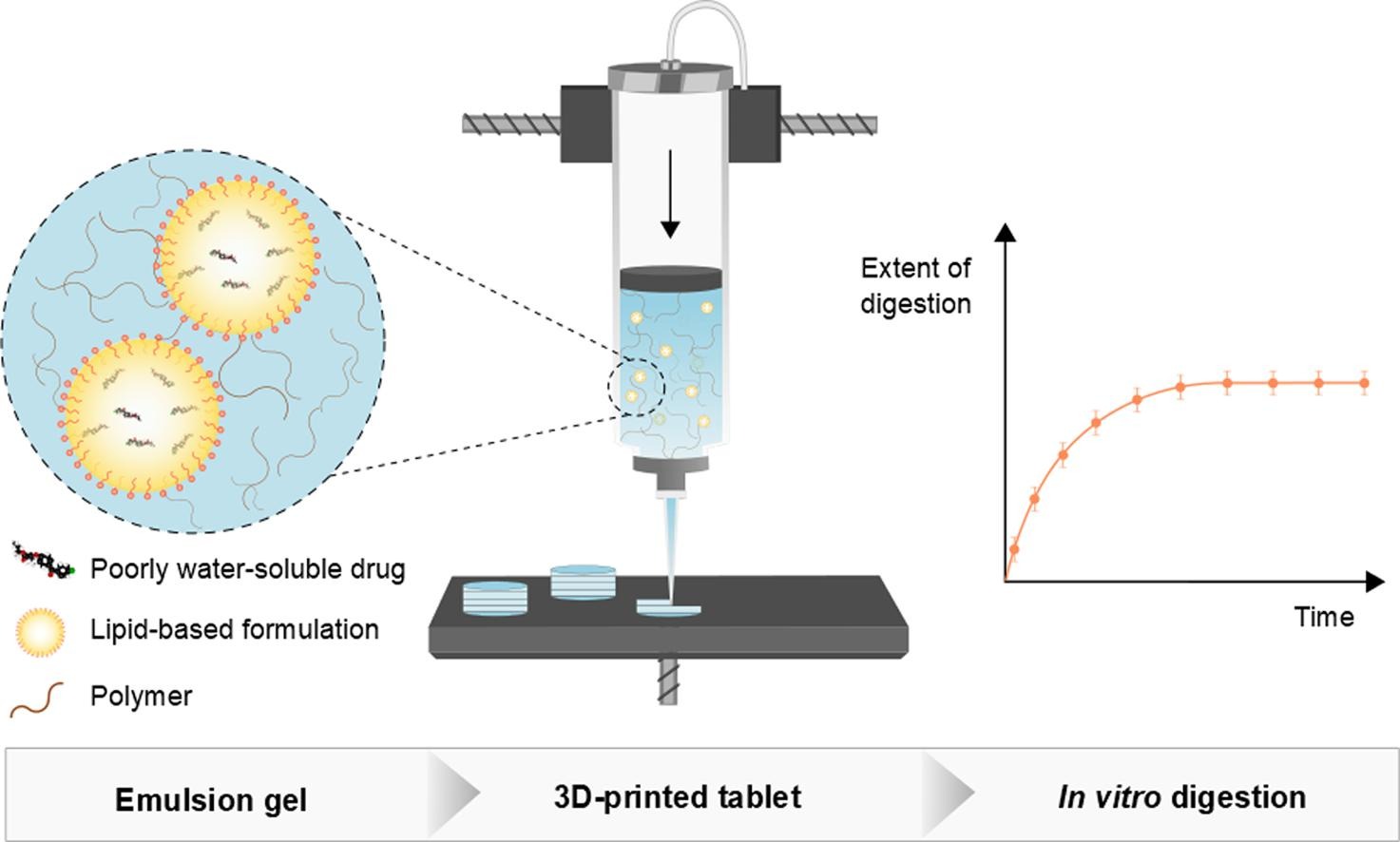
Interest in 3D-printing technologies for pharmaceutical manufacturing of oral dosage forms is driven by the need for personalized medicines. Most research to date has focused on printing of polymeric-based drug delivery systems at high temperatures. Furthermore, oral formulation development is continuously challenged by the large number of poorly water-soluble drugs, which require more advanced enabling formulations to improve oral bioavailability. In this work, we used semi-solid extrusion (SSE) printing of emulsion gels with three types of emulsified lipid-based formulations (LBFs) to produce solid lipid tablets incorporating the poorly water-soluble drug, fenofibrate. Tablets were successfully 3D-printed from emulsion gels using SSE at room temperature, making the methodology particularly useful for thermolabile compounds. The tablets were well-defined in mass and disintegrated rapidly (<15 min). Importantly, the oil droplet size reconstituted after dispersion of the tablets and subsequent lipid digestion was similar to traditional liquid LBFs. This work demonstrates the successful use of SSE for fabricating solid lipid tablets based on emulsion gels. The method is further promising for on demand production of personalized dosage forms, necessary for flexible dosage adjustment in e.g., pediatric patients, when poorly water-soluble compounds constitute the core of the therapy.
Download the full article here: 3D-printing of solid lipid tablets from emulsion gels
or continue reading here: Jenny Johannesson, Jamal Khan, Madlen Hubert, Alexandra Teleki, Christel A.S. Bergström, 3D-printing of solid lipid tablets from emulsion gels, International Journal of Pharmaceutics,Volume 597,
2021,120304, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2021.120304.(https://www.sciencedirect.com/science/article/pii/S0378517321001083)
Materials
Maisine CC (mixed long-chain glycerides) was kindly provided by Gattefossé (Lyon, France), Captex 355 EP/NF (medium-chain triglycerides) and Capmul MCM EP (mixed long-chain glycerides) by Abitec (Janesville, USA). Soybean oil (long-chain triglycerides), Kolliphor EL (polyethoxylated castor oil) and Tween 85, fenofibrate (≥99%), methyl cellulose (Methocel A4M), Nile red, porcine pancreatin extract (8 × USP specifications activity), 4-bromophenylboronic acid, trizma maleate, calcium chloride dihydrate, sodium hydroxide pellets, sodium acetate, acetic acid, silica gel, acetonitrile (≥99.9%) and 2-propanol (99.9%) were obtained from Merck (Darmstadt, Germany). Fasted-state simulated intestinal fluid (FaSSIF) powder was purchased from biorelevant.com (Croydon, UK) and croscarmellose sodium (Ac-Di-Sol) from FMC (Brussels, Belgium).
Find our full pharmaceutical 3D printing overview article here
OR
Visit our 3D printing special here
Are you interested to know more about Pharmaceutical 3D Printing?
Then two speeches of the ExciPerience might be of big interest for you!



