Carboxymethyl-β-glucan/chitosan nanoparticles: new thermostable and efficient carriers for antigen delivery
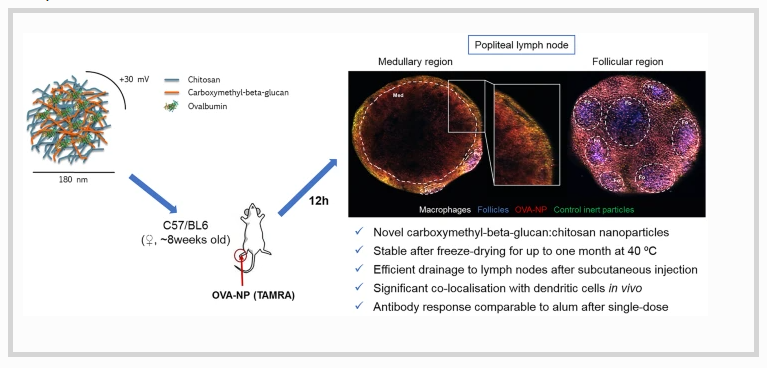
In the last few decades, nanotechnology has emerged as an important tool aimed at enhancing the immune response against modern antigens. Nanocarriers designed specifically for this purpose have been shown to provide protection, stability, and controlled release properties to proteins, peptides, and polynucleotide-based antigens. Polysaccharides are particularly interesting biomaterials for the construction of these nanocarriers given their wide distribution among pathogens, which facilitates their recognition by antigen-presenting cells (APCs). In this work, we focused on an immunostimulant beta-glucan derivative, carboxymethyl-β-glucan, to prepare a novel nanocarrier in combination with chitosan.
The resulting carboxymethyl-β-glucan/chitosan nanoparticles exhibited adequate physicochemical properties and an important protein association efficiency, with ovalbumin as a model compound. Moreover, thermostability was achieved through the optimization of a lyophilized form of the antigen-loaded nanoparticles, which remained stable for up to 1 month at 40 ºC. Biodistribution studies in mice showed an efficient drainage of the nanoparticles to the nearest lymph node following subcutaneous injection, and a significant co-localization with dendritic cells. Additionally, subcutaneous immunization of mice with a single dose of the ovalbumin-loaded nanoparticles led to induced T cell proliferation and antibody responses, comparable to those achieved with alum-adsorbed ovalbumin. These results illustrate the potential of these novel nanocarriers in vaccination.
Download the full research paper as pdf: Carboxymethyl‑β‑glucanchitosan nanoparticles new thermostable and efficient carriers for antigen delivery
Cordeiro, A.S., Farsakoglu, Y., Crecente-Campo, J. et al. Carboxymethyl-β-glucan/chitosan nanoparticles: new thermostable and efficient carriers for antigen delivery. Drug Deliv. and Transl. Res. (2021). https://doi.org/10.1007/s13346-021-00968-9
Materials
Pharmaceutical grade chitosan hydrochloride, with a molecular weight of 47 kDa and an 80–95% deacetylation degree, was acquired from Heppe Medical Chitosan GmbH (Halle, Germany). Carboxymethyl-β-glucan, obtained from Saccharomyces cerevisae and modified with carboxymethyl groups at an 85% substitution degree, was a kind donation from Mibelle AG Biochemistry (Buchs, Switzerland). Ovalbumin, in the form of complete protein with a molecular weight of 45 kDa, was provided by Invivogen (Toulouse, France). 5-Carboxytetramethyl-rhodamine succinimidyl ester (5-TAMRA SE) was acquired from emp Biotech GmbH (Berlin, Germany). Sucrose dilaureate (Surfhope® D1216) was obtained from Mitsubishi-Chemical Foods Corporation (Tokyo, Japan). Sucrose was bought from Acofarma (Madrid, Spain) and trehalose (pharmaceutical grade) from Pfanstiehl (Waukegan (IL), USA). Polyvinyl pyrrolidone (PVP, Kollidon® 25) was purchased from BASF (Ludwigshafen, Germany). Squalene was acquired from Merck Millipore (Darmstadt, Germany). Sorbitan monooleate (Span® 80), bovine serum albumine, and glycine were obtained from Sigma-Aldrich (Saint Louis, USA). All solvents were of HPLC grade, supplied by Thermo Fisher Scientific (Waltham, USA) or Scharlab SL (Barcelona, Spain).

