Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability
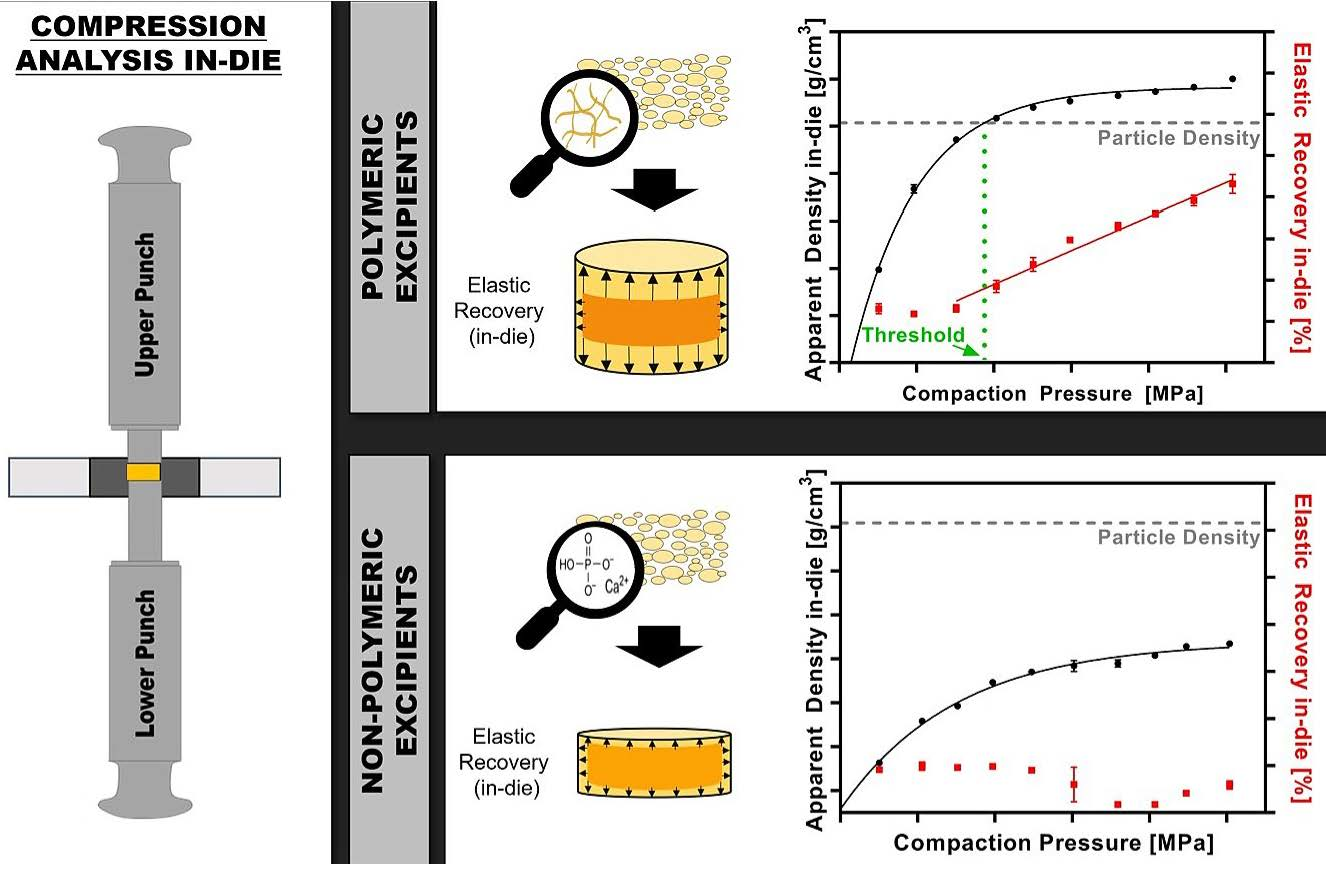
The present study focuses on the compaction behavior of polymeric excipients during compression in comparison to nonpolymeric excipients and its consequences on commonly used Heckel analysis. Compression analysis at compaction pressures (CPs) from 50 to 500 MPa was performed using a compaction simulator. This study demonstrates that the particle density, measured via helium pycnometer (ρpar), of polymeric excipients (Kollidon®VA64, Soluplus®, AQOAT®AS-MMP, Starch1500®, Avicel®PH101) was already exceeded at low CPs (<200 MPa), whereas the ρpar was either never reached for brittle fillers such as DI-CAFOS®A60 and tricalcium citrate or exceeded at CPs above 350 MPa (FlowLac®100, Pearlitol®100SD). We hypothesized that the threshold for exceeding ρpar is linked with predominantly elastic deformation. This was confirmed by the start of linear increase in elastic recovery in-die (ERin-die) with exceeding particle density, and in addition, by the applicability in calculating the elastic modulus via the equation of the linear increase in ERin-die. Last, the evaluation of “density under pressure” as an alternative to the ρpar for Heckel analysis showed comparable conclusions for compression behavior based on the calculated yield pressures. However, the applicability of Heckel analysis for polymeric excipients was questioned in principle. In conclusion, the knowledge of the threshold provides guidance for the selection of suitable excipients in the formulation development to mitigate the risk of tablet defects related to stored elastic energy, such as capping and lamination.
Continue reading here
About this article: Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability. Pharmaceutics 2022, 14, 913. https://doi.org/10.3390/pharmaceutics14050913
Materials
Di-calcium phosphate (DI-CAFOS®A60) was obtained from Chemische Fabrik Budenheim (Budenheim, Germany), tricalcium citrate tetrahydrate (TriCaCi) from Jungbunzlauer Ladenburg GmbH (Ladenburg, Germany), microcrystalline cellulose (Avicel®PH101) from FMC (Philadelphia, PA, USA), alpha-lactose monohydrate (FlowLac®100) from Meggle Group (Wasserburg, Germany), mannitol (Pearlitol®100SD) from Roquette GmbH (Frankfurt a. M., Germany), copovidone (polyvinylpyrrolidone-vinyl acetate copolymer, Kollidon®VA 64) and polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®) from BASF SE (Ludwigshafen, Germany), hypromellose acetate succinate (HPMCAS, AQOAT®AS-MMP) from Shin-Etsu Chemical Co. Ltd. (Tokyo, Japan), and partially pregelatinized maize starch (Starch1500®) from Colorcon Limited (Kent, UK).
DI-CAFOS®A60 are aggregates of fine, almost spherical particles with an uneven surface and a d50 value of 60 µm [30]. TriCaCi is a powder of almost spherically shaped large agglomerates with a mean particle size of 135 µm [26]. Avicel®PH101 consists of irregularly shaped particles with a broad particle size distribution and a mean particle size of approximately 56 µm [31,32]. FlowLac®100 is manufactured via spray drying, which explains the spherical shape of the particles with a mean particle size of 110 µm [33]. In addition, Pearlitol®100SD is prepared via spray drying resulting in spherical particles with a mean particle size of 100 µm [34]. The mean particle size of Kollidon®VA 64 is 82 µm, and the particles are hollow spheres in a significant proportion [35]. Soluplus® appears as particles with a diameter of approximately 340 microns in a mostly spherical shape, according to the technical information of the vendor [36]. AQOAT®AS-MMP consists of particles with a mean particle size of approximately 300 µm. Starch1500® consists of particles with a broad particle size distribution and a mean particle size of approximately 65 µm [37].
The amorphous solid dispersion (ASD) consists of 15% (w/w) ritonavir, 74% (w/w) copovidone, 10% (w/w) sorbitan monolaurate, and 1% (w/w) silicon dioxide, and the tablet blend of 87.1% (w/w) milled extrudate (ASD), 11.7% (w/w) di-calcium phosphate, 0.9% (w/w) silicon dioxide, and 0.3% (w/w) sodium stearyl fumarate. Both ASD and tablet blend composition were in accordance with the marketed Norvir® formulation serving as model ASD formulation in the present study representative for other ASDs. The ASD was manufactured by hot-melt extrusion and was kindly provided by AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany.
In detail, ritonavir (purity > 99.8%) was obtained from AbbVie Inc. (North Chicago, IL, USA) and sorbitan monolaurate (Span20®) from CRODA (Nettetal, Germany). Di-calcium phosphate (DI-CAFOS® A60) was purchased from Chemische Fabrik Budenheim (Budenheim, Germany), fumed silicon dioxide (Aerosil®200) from Evonik Industries (Essen, Germany), and sodium stearyl fumarate (PRUV®) from JRS Pharma (Rosenberg, Germany).
To ease the readability of the figures within the article, the respective excipients were displayed without their trademarks.

