Commercially Available Enteric Empty Hard Capsules, Production Technology and Application
Currently, there is a growing need to prepare small batches of enteric capsules for individual therapy or clinical evaluation since many acidic-sensitive substances should be protected from the stomach’s acidic environment, including probiotics or fecal material, in the fecal microbiota transplantation (FMT) process. A suitable method seems to be the encapsulation of drugs or lyophilized alternatively frozen biological suspensions in commercial hard enteric capsules prepared by so-called Enteric Capsule Drug Delivery Technology (ECDDT).
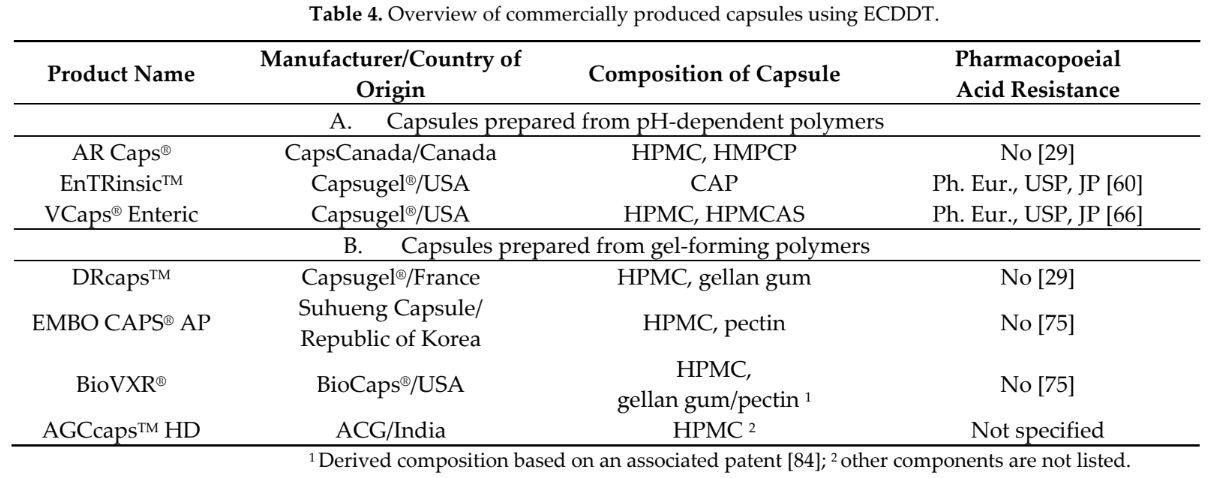
Manufacturers supply these types of capsules, made from pH-soluble polymers, in products such as AR Caps®, EnTRinsicTM, and Vcaps® Enteric, or capsules made of gelling polymers that release their content as the gel erodes over time when passing through the digestive tract. These include DRcaps®, EMBO CAPS® AP, BioVXR®, or ACGcaps™ HD. Although not all capsules in all formulations meet pharmaceutical requirements for delayed-release dosage forms in disintegration and dissolution tests, they usually find practical application. This literature review presents their composition and properties. Since ECDDT is a new technology, this article is based on a limited number of references.
Download the full study as PDF here Commercially Available Enteric Empty Hard Capsules, Production Technology and Application
or read it here
Exicpient mentioned in the study besides other: HPMC, Eudragit® FS, Eudragit® S, Eudragit® L 100, Eudragit® FL, Tween 80, starch, PVA, sodium alginate
Franc, A.; Vetchý, D.; Fülöpová, N. Commercially Available Enteric Empty Hard Capsules, Production Technology and Application. Pharmaceuticals 2022, 15, 1398.
https://doi.org/10.3390/ph15111398
See also the newest development in capsule, presented at CPhI 2022:
The Capsugel® Enprotect™ capsule


