Overcoming Challenges in Ophthalmic Formulations through Polymer Selection – A Closer Look at Polyvinyl Alcohol
Ophthalmic drug formulations are growing in importance due to the increased prevalence of eye-related disorders such as diabetic retinopathy and macular degeneration.1 However, ocular drug delivery is challenging due to unique anatomical and physiological barriers such as pre-corneal loss factors including tear turnover, nasolacrimal drainage with potential systemic absorption via the conjunctiva or nasal mucosa, transient residence time, and the relative impermeability of the corneal epithelial membrane.2 The low ocular bioavailability (<10%) of conventional ophthalmic formulations is driving the need for novel approaches to improve delivery of the desired concentration, at the site of action, at a controlled rate.3
formulation challenges presented by ophthalmics.
The formulation of ophthalmic drugs must address a unique combination of requirements. In addition to ensuring quality, drug tolerability and fostering patient compliance, formulators must also consider tonicity, viscosity, pH, stability, sterility and microbiological purity. Further, pharmacopoeias state that ophthalmic solutions must be essentially free from particles that can be observed on visual inspection.
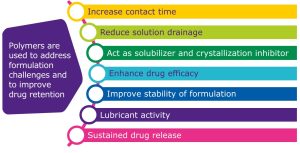
Polymers are an important part of the formulation toolbox and offer several benefits for ophthalmic dosage forms (Figure 1). They can increase contact time with the target tissue and reduce drainage of the solution, helping to enhance efficacy of the drug. If viscosity is initially too low, polymers can help increase it to sustain release of the active pharmaceutical ingredient (API). They can act as solubilizers, inhibit crystallization, stabilize the formulation, and serve as a lubricant.
This white paper by Merck provides an overview of polymers that can be used in ophthalmic formulations and highlights advantages offered using polyvinyl alcohol (PVA) through case studies.
Considerations for Polymer Selection
In addition to selecting the right polymer for the formulation, aspects related to preparing the polymer solution, sterilization and interaction with other excipients in the final formulation must be considered.
A variety of polymers can be used in ophthalmic formulations including those of natural, synthetic and semi-synthetic origins. Natural polymers such as gellan, xanthan, and guar gum, and hyaluronic acid are relatively inconsistent in terms of viscosity and have the potential for a higher microbial load as compared to semi-synthetic and synthetic polymers. Control over the microbial load can be challenging and for an ophthalmic formulation, there is a stringent limit to what is allowed. Semi-synthetic polymers have a higher probability of batch-to-batch variation and broader range of viscosity which can impact performance. Examples include hydroxypropyl methylcellulose (HPMC), carboxymethyl cellulose (CMC), and hydroxyethyl cellulose (HEC). The advantages of synthetic polymers include high batch to batch consistency and a narrow range of viscosity which helps to deliver a reproducible performance. Examples of synthetic polymers are polyvinyl alcohol (PVA), carbomer and polyvinyl pyrrolidone (PVP).
Following selection of the polymer, the next step is preparation of the solution. Preparation of bulk-quantity polymer solutions for scale-up or commercial manufacturing can be time-consuming. For some polymers, heating is required for dissolution, and this necessitates use of jacketed vessels as well as additional time needed for heat-up of the solvent and cool-down of the polymer solution. If the polymer is not properly dissolved, the final concentration will be impacted. Also, in this process step the polymer choice is very important: if a polymer of insufficient quality and purity is used, insoluble impurities may be encountered in the polymer solution. Removal of these undissolved particles is mandatory to meet the quality expectations for ophthalmic preparations such as the pharmacopeial requirement for particle-free eye drops.
After preparing the polymer solution, it must be sterilized. Selection of the sterilization method, which can include steam or filtration, is critical as it must be compatible with the polymer solution. The method should not have an impact on the critical quality attributes of the polymer such as viscosity or the molecular weight. Preparation of the final formulation can also present challenges. The potential for interaction of the polymers with other excipients throughout the shelf life and any impact on stability must be understood.
PVA: A Versatile Excipient for Ophthalmics
PVA (sometimes referred to as PVOH) is a biocompatible synthetic polymer produced by the polymerization of vinyl acetate and partial hydrolysis of the resulting esterified polymer. PVA has been used in approved drug products for decades and has a long history of use in the food and cosmetic industries. It is generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA),4 does not have any immunogenic effects, and its long-term use has been demonstrated in many different formulations including oral, topical and ophthalmic.
PVA was first used in ophthalmics in the 1960s to increase solution viscosity and prolong precorneal residence time.5 Incorporation of PVA in ophthalmic preparations significantly delays precorneal drainage of locally applied formulations, leading to an improved therapeutic effect.
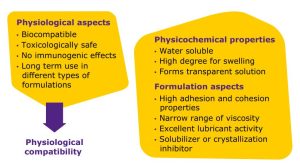
PVA offers many advantages for ophthalmic formulations (Figure 2). It is water soluble, has a narrow range of viscosity, and a high degree of swelling, offering the precise viscosity needed for formulations to remain in the eye cavity. This polymer also forms a transparent solution which is important for medications administered to the eye, as is high adhesion and high correlation properties, which are also important for retention in the eye cavity. With excellent lubricant activity, this polymer is well-suited for lubricating eye drops. Finally, PVA acts an inhibitor of crystallization which means it helps retain solubility of the API throughout storage of the dosage form.
formulations.
PVA Selection
PVAs are available in different grades based on viscosity, hydrolysis and the microbial load (total aerobic bacteria, total yeast and total mold count), which should be specified by the manufacturer. Another important consideration when using PVA is the possible presence of crotonaldehyde, which is a process impurity generated during synthesis. While there is no information in the pharmacopeia regarding the limit of this impurity, given its toxic nature, it is important that this impurity content is known and controlled. Due to stringent regulatory requirements in this segment, a multi-compendial product and regulatory and documentation support by the manufacturer is desirable to facilitate regulatory submission. Again, the selection of the right polymer is critical: with PVA, there are different grades available on the market and the choice will affect the final product performance.
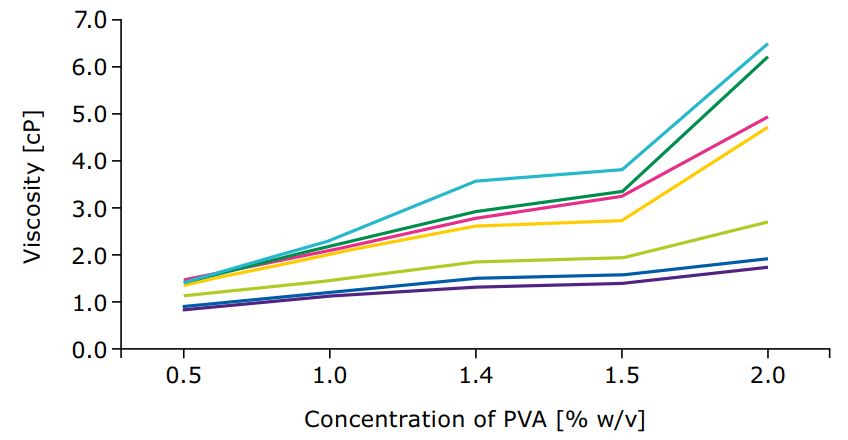
Different grades come with different physicochemical properties, making them suitable for different applications. Lower viscosity grades of PVA can be used for lubricant activity, to enhance API solubility and as inhibitors of crystallization. If the formulation is a suspension or gel, a high viscosity grade PVA is more suitable and can be used as a thickener or viscosity enhancer. Different hydrolysis grades of PVA, which refers to the amount of residual, unhydrolyzed acetate groups within the polymer chain, also affect the polymer performance. Higher hydrolysis grades improve tensile strength of the hydrogel and provide a stronger gel scaffold through H-bonds while lower hydrolysis grades might be a better choice for drug delivery of poorly water-soluble APIs. However, since some pharmacopoeias restrict the hydrolysis grade variation, there is limited flexibility in this aspect.
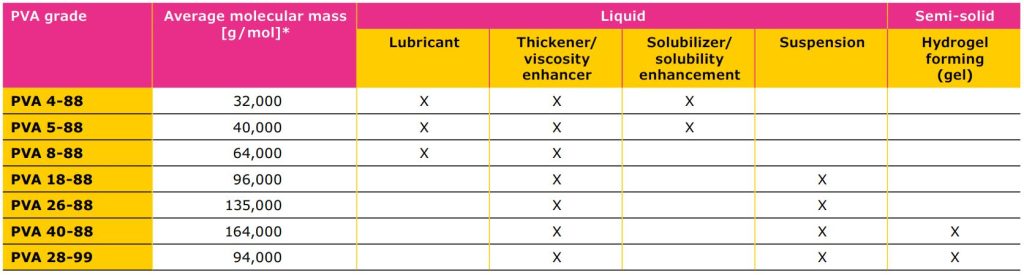
specifies the apparent viscosity in mPa• s of a 4% aqueous solution at 20 °C (first number) and the hydrolysis grade (second number).
PVA Solution Preparation
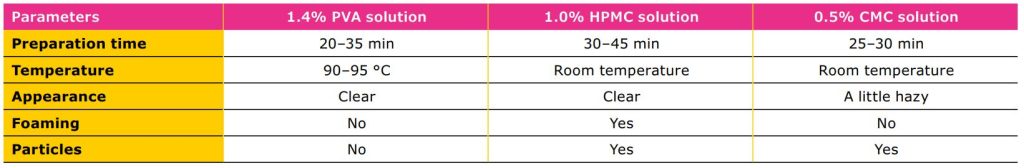
Table 2 compares the preparation, appearance,
and presence of foam and particles in solutions of
three different polymers: PVA, HPMC, and CMC. The
selected concentrations reflect those commonly used
in ophthalmic preparations. While the temperature
requirement for solubilization of PVA was relatively
high, the solution was clear with no foaming or
particles (visually observed). In PVA handling, a higher
temperature must be applied for dissolution processes.
Having considered this aspect, it is easy to obtain a
clear, particle free solution without foam. While HPMC
and CMC can be dissolved at room temperature, it may
be challenging to obtain particle-free solutions.
The Advantages of PVA
The data presented in this white paper confirm that PVA meets all the essential requirements to be used in ophthalmic preparations and offers important benefits compared to HPMC and CMC (Table 4).
As they are semi-synthetic in nature, HPMC and CMC have a relatively large range in viscosity compared to PVA and can be expected to have a higher microbial load. While PVA requires a higher temperature for solution preparation, foam and particles have been observed in HPMC and CMC solutions.
PVA and HPMC can be sterile filtered while CMC cannot. PVA has a high throughput, and the filter surface area required is relatively low compared to that needed to process an HPMC solution. No changes were observed in the viscosity of PVA and HPMC following sterile filtration; the viscosity of both was also stable following steam sterilization while changes were observed in the viscosity of CMC. Changes in pH did not affect the PVA solution and this polymer was shown to be compatible with a variety of inorganic salts.
Ophthalmic medications play a critical role in the treatment of many diseases and conditions and their use continues to expand. The unique route of administration imposes stringent requirements for the final formulation to ensure a success drug product. While several polymers are available for use in ophthalmic formulations, PVA offers important advantages, is best suited to overcome many formulation challenges, and should be considered as an alternative to HPMC and CMC.
See the full white paper on “Overcoming Challenges in Ophthalmic Formulations through Polymer Selection by Merck“:
(click the picture to download the brochure)
Source: Merck brochure “Overcoming Challenges in Ophthalmic Formulations through Polymer Selection by Merck”
Do you need more information or a sample of excipients by Merck?


