Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process
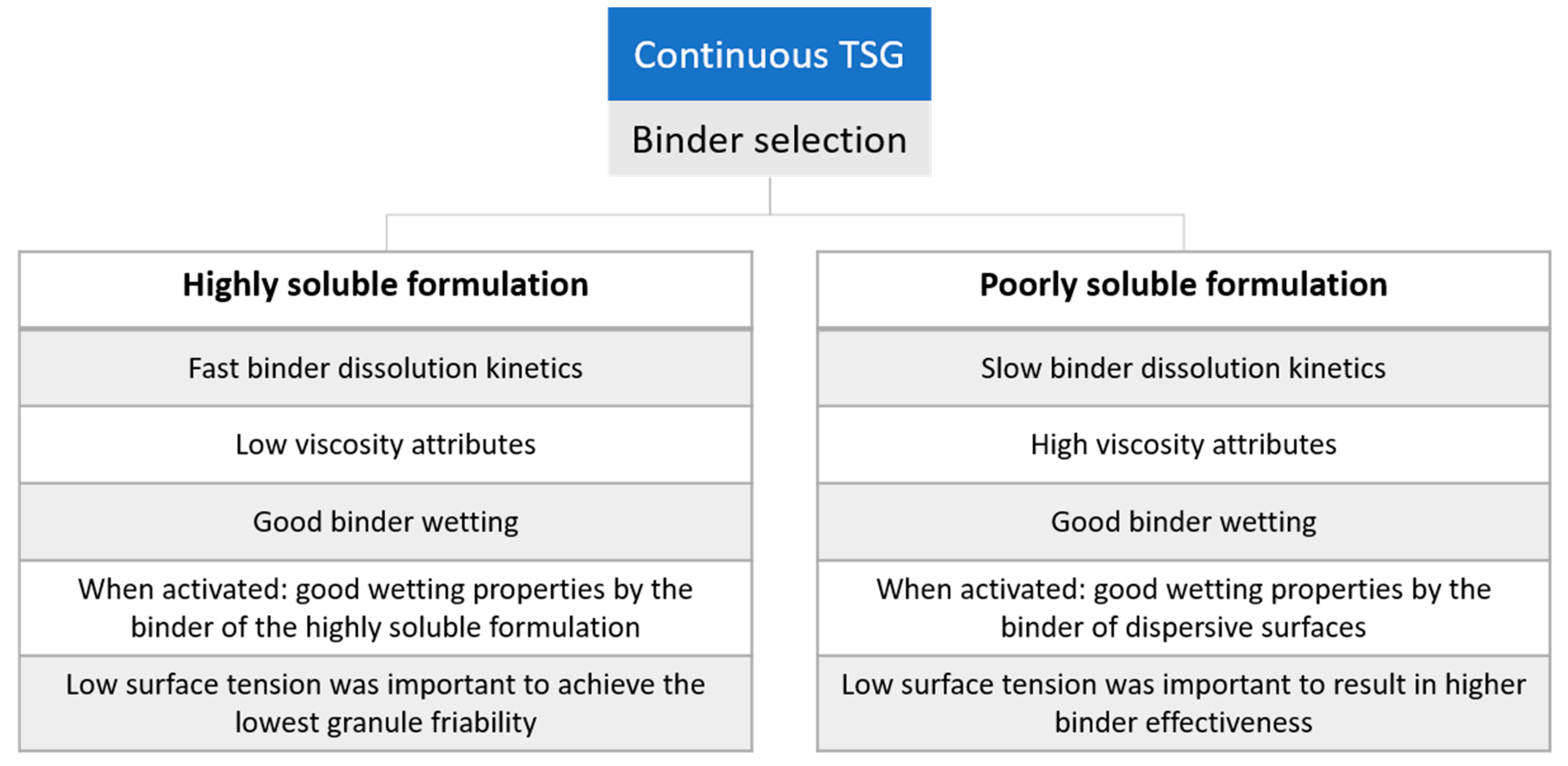
The suitability of pharmaceutical binders for continuous twin-screw wet granulation was investigated as the pharmaceutical industry is undergoing a switch from batch to continuous manufacturing. Binder selection for twin-screw wet granulation should rely on a scientific approach to enable efficient formulation development. Therefore, the current study identified binder attributes affecting the binder effectiveness in a wet granulation process of a highly soluble model excipient (mannitol). For this formulation, higher binder effectiveness was linked to fast activation of the binder properties (i.e., fast binder dissolution kinetics combined with low viscosity attributes and good wetting properties by the binder). As the impact of binder attributes on the granulation process of a poorly soluble formulation (dicalcium phosphate) was previously investigated, this enabled a comprehensive comparison between both formulations in current research focusing on binder selection. This comparison revealed that binder attributes that are important to guide binder selection differ in function of the solubility of the formulation. The identification of critical binder attributes in the current study enables rational and efficient binder selection for twin-screw granulation of well soluble and poorly soluble formulations. Binder addition proved especially valuable for a poorly soluble formulation.
Download the full publication here: Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin ScrewWet Granulation Process
or continue reading here: Vandevivere, L.; Vangampelaere, M.; Portier, C.; de Backere, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Identifying Critical Binder Attributes to Facilitate Binder Selection for Efficient Formulation Development in a Continuous Twin Screw Wet Granulation Process. Pharmaceutics 2021, 13, 210. https://doi.org/10.3390/pharmaceutics13020210

